Anti-gp41 antibodies cloned from HIV-infected patients with broadly neutralizing serologic activity
- PMID: 20219932
- PMCID: PMC2863839
- DOI: 10.1128/JVI.00154-10
Anti-gp41 antibodies cloned from HIV-infected patients with broadly neutralizing serologic activity
Abstract
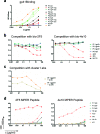
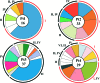
Most HIV-infected individuals develop antibodies to the gp120 and gp41 components of the viral spike; however, only a fraction of these individuals mount a broadly neutralizing serum response against HIV. We have cloned anti-HIV antibodies from the memory B-cell compartment of six individuals with variable viral loads and high titers of broadly neutralizing antibodies. Here, we report on the features of the anti-gp41 response in these patients. Competition experiments with previously characterized antibodies targeting defined epitopes on the gp41 ectodomain showed antibodies directed against the "immunodominant region" (cluster I), the carboxy-terminal heptad repeat (cluster II), and the membrane-proximal external region (cluster IV). On the other hand, antibodies directed against the amino-terminal part of the molecule, including the fusion peptide, polar region, and the N-terminal heptad repeat, were not detected. When all patients' data were combined, unique B-cell clones targeting cluster I, II, and IV accounted for 32%, 49%, and 53% of all anti-gp41-reactive B cells, respectively; therefore, no single region was truly immunodominant. Finally, although we found no new neutralizing epitopes or HIV-1-neutralizing activity by any of the gp41 antibodies at concentrations of up to 50 microg/ml, high concentrations of 7 out of 15 anti-cluster I antibodies neutralized tier 2 viruses.
Figures
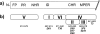
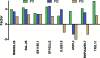


Similar articles
-
Amino Acid Changes in the HIV-1 gp41 Membrane Proximal Region Control Virus Neutralization Sensitivity.EBioMedicine. 2016 Oct;12:196-207. doi: 10.1016/j.ebiom.2016.08.045. Epub 2016 Aug 31. EBioMedicine. 2016. PMID: 27612593 Free PMC article.
-
Identification of HIV gp41-specific antibodies that mediate killing of infected cells.PLoS Pathog. 2019 Feb 19;15(2):e1007572. doi: 10.1371/journal.ppat.1007572. eCollection 2019 Feb. PLoS Pathog. 2019. PMID: 30779811 Free PMC article.
-
Anti-V3/Glycan and Anti-MPER Neutralizing Antibodies, but Not Anti-V2/Glycan Site Antibodies, Are Strongly Associated with Greater Anti-HIV-1 Neutralization Breadth and Potency.J Virol. 2015 May;89(10):5264-75. doi: 10.1128/JVI.00129-15. Epub 2015 Feb 11. J Virol. 2015. PMID: 25673728 Free PMC article.
-
Towards an AIDS vaccine: the transmembrane envelope protein as target for broadly neutralizing antibodies.Hum Vaccin. 2011 Jan-Feb;7 Suppl:4-9. doi: 10.4161/hv.7.0.14555. Epub 2011 Jan 1. Hum Vaccin. 2011. PMID: 21266839 Review.
-
B cell responses to HIV and the development of human monoclonal antibodies.Clin Exp Immunol. 1992 May;88(2):189-202. doi: 10.1111/j.1365-2249.1992.tb03061.x. Clin Exp Immunol. 1992. PMID: 1572084 Free PMC article. Review.
Cited by
-
Rational design of membrane proximal external region lipopeptides containing chemical modifications for HIV-1 vaccination.Clin Vaccine Immunol. 2013 Jan;20(1):39-45. doi: 10.1128/CVI.00615-12. Epub 2012 Oct 31. Clin Vaccine Immunol. 2013. PMID: 23114698 Free PMC article.
-
Pre-clinical evaluation of a 213Bi-labeled 2556 antibody to HIV-1 gp41 glycoprotein in HIV-1 mouse models as a reagent for HIV eradication.PLoS One. 2012;7(3):e31866. doi: 10.1371/journal.pone.0031866. Epub 2012 Mar 9. PLoS One. 2012. PMID: 22427811 Free PMC article.
-
Significance of Conserved Regions in Coronavirus Spike Protein for Developing a Novel Vaccine against SARS-CoV-2 Infection.Vaccines (Basel). 2023 Feb 24;11(3):545. doi: 10.3390/vaccines11030545. Vaccines (Basel). 2023. PMID: 36992129 Free PMC article. Review.
-
Candidate antibody-based therapeutics against HIV-1.BioDrugs. 2012 Jun 1;26(3):143-62. doi: 10.2165/11631400-000000000-00000. BioDrugs. 2012. PMID: 22462520 Free PMC article. Review.
-
Enhanced HIV-1 neutralization by antibody heteroligation.Proc Natl Acad Sci U S A. 2012 Jan 17;109(3):875-80. doi: 10.1073/pnas.1120059109. Epub 2012 Jan 4. Proc Natl Acad Sci U S A. 2012. PMID: 22219363 Free PMC article.
References
-
- Barin, F., M. F. McLane, J. S. Allan, T. H. Lee, J. E. Groopman, and M. Essex. 1985. Virus envelope protein of HTLV-III represents major target antigen for antibodies in AIDS patients. Science 228:1094-1096. - PubMed
-
- Binley, J. M., E. A. Lybarger, E. T. Crooks, M. S. Seaman, E. Gray, K. L. Davis, J. M. Decker, D. Wycuff, L. Harris, N. Hawkins, B. Wood, C. Nathe, D. Richman, G. D. Tomaras, F. Bibollet-Ruche, J. E. Robinson, L. Morris, G. M. Shaw, D. C. Montefiori, and J. R. Mascola. 2008. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J. Virol. 82:11651-11668. - PMC - PubMed
-
- Binley, J. M., H. J. Ditzel, C. F. Barbas, N. Sullivan, J. Sodroski, P. W. Parren, and D. R. Burton. 1996. Human antibody responses to HIV type 1 glycoprotein 41 cloned in phage display libraries suggest three major epitopes are recognized and give evidence for conserved antibody motifs in antigen binding. AIDS Res. Hum. Retroviruses 12:911-924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

