Preclinical studies of a modified vaccinia virus Ankara-based HIV candidate vaccine: antigen presentation and antiviral effect
- PMID: 20219934
- PMCID: PMC2863849
- DOI: 10.1128/JVI.02329-09
Preclinical studies of a modified vaccinia virus Ankara-based HIV candidate vaccine: antigen presentation and antiviral effect
Abstract
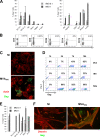
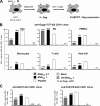
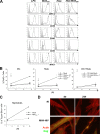
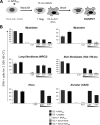
Poxvirus-based human immunodeficiency virus (HIV) vaccine candidates are currently under evaluation in preclinical and clinical trials. Modified vaccinia virus Ankara (MVA) vectors have excellent safety and immunogenicity records, but their behavior in human cell cultures remains only partly characterized. We studied here various virological and immunological aspects of the interactions of MVA-HIV, a vaccine candidate developed by the French National Agency for AIDS Research (ANRS), with primary human cells. We report that MVA-HIV infects and drives Gag expression in primary macrophages, dendritic cells (DCs), and epithelial and muscle cells. MVA-HIV-infected DCs matured, efficiently presented Gag, Pol, and Nef antigens, and activated HIV-specific cytotoxic T lymphocytes (CTLs). As expected with this type of vector, infection was cytopathic and led to DC apoptosis. Coculture of MVA-HIV-infected epithelial cells or myotubes with DCs promoted efficient Gag antigen major histocompatibility complex class I (MHC-I) cross-presentation without inducing direct infection and death of DCs. Antigen-presenting cells (APCs) infected with MVA-HIV also activated HIV-specific CD4(+) T cells. Moreover, exposure of DCs to MVA-HIV or to MVA-HIV-infected myotubes induced type I interferon (IFN) production and inhibited subsequent HIV replication and transfer to lymphocytes. Altogether, these results show that MVA-HIV promotes efficient MHC-I and MHC-II presentation of HIV antigens by APCs without facilitating HIV replication. Deciphering the immune responses to MVA in culture experiments will help in the design of innovative vaccine strategies.
Figures


Similar articles
-
MVA-nef induces HIV-1-specific polyfunctional and proliferative T-cell responses revealed by the combination of short- and long-term immune assays.Gene Ther. 2010 Nov;17(11):1372-83. doi: 10.1038/gt.2010.90. Epub 2010 Jun 10. Gene Ther. 2010. PMID: 20535218
-
Modified vaccinia virus Ankara-infected dendritic cells present CD4+ T-cell epitopes by endogenous major histocompatibility complex class II presentation pathways.J Virol. 2015 Mar;89(5):2698-709. doi: 10.1128/JVI.03244-14. Epub 2014 Dec 17. J Virol. 2015. PMID: 25520512 Free PMC article.
-
Improving recombinant MVA immune responses: potentiation of the immune responses to HIV-1 with MVA and DNA vectors expressing Env and the cytokines IL-12 and IFN-gamma.Virus Res. 2006 Mar;116(1-2):11-20. doi: 10.1016/j.virusres.2005.08.008. Epub 2005 Oct 7. Virus Res. 2006. PMID: 16214252
-
Attenuated poxvirus vectors MVA and NYVAC as promising vaccine candidates against HIV/AIDS.Hum Vaccin. 2009 Dec;5(12):867-71. doi: 10.4161/hv.9693. Epub 2009 Dec 3. Hum Vaccin. 2009. PMID: 19786840 Review.
-
Clinical applications of attenuated MVA poxvirus strain.Expert Rev Vaccines. 2013 Dec;12(12):1395-416. doi: 10.1586/14760584.2013.845531. Epub 2013 Oct 30. Expert Rev Vaccines. 2013. PMID: 24168097 Review.
Cited by
-
Gene Expression Driven by a Strong Viral Promoter in MVA Increases Vaccination Efficiency by Enhancing Antibody Responses and Unmasking CD8⁺ T Cell Epitopes.Vaccines (Basel). 2014 Jul 22;2(3):581-600. doi: 10.3390/vaccines2030581. Vaccines (Basel). 2014. PMID: 26344747 Free PMC article.
-
A novel high-throughput vaccinia virus neutralization assay and preexisting immunity in populations from different geographic regions in China.PLoS One. 2012;7(3):e33392. doi: 10.1371/journal.pone.0033392. Epub 2012 Mar 16. PLoS One. 2012. PMID: 22438922 Free PMC article.
-
HIV-1 T cell epitopes targeted to Rhesus macaque CD40 and DCIR: A comparative study of prototype dendritic cell targeting therapeutic vaccine candidates.PLoS One. 2018 Nov 30;13(11):e0207794. doi: 10.1371/journal.pone.0207794. eCollection 2018. PLoS One. 2018. PMID: 30500852 Free PMC article.
-
S100A9 Tetramers, Which are Ligands of CD85j, Increase the Ability of MVAHIV-Primed NK Cells to Control HIV Infection.Front Immunol. 2015 Sep 23;6:478. doi: 10.3389/fimmu.2015.00478. eCollection 2015. Front Immunol. 2015. PMID: 26441983 Free PMC article.
-
Modified vaccinia virus Ankara-based vaccine vectors induce apoptosis in dendritic cells draining from the skin via both the extrinsic and intrinsic caspase pathways, preventing efficient antigen presentation.J Virol. 2012 May;86(10):5452-66. doi: 10.1128/JVI.00264-12. Epub 2012 Mar 14. J Virol. 2012. PMID: 22419811 Free PMC article.
References
-
- Amara, R. R., F. Villinger, S. I. Staprans, J. D. Altman, D. C. Montefiori, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Different patterns of immune responses but similar control of a simian-human immunodeficiency virus 89.6P mucosal challenge by modified vaccinia virus Ankara (MVA) and DNA/MVA vaccines. J. Virol. 76:7625-7631. - PMC - PubMed
-
- Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385. - PubMed
-
- Bevan, M. J. 2004. Helping the CD8(+) T-cell response. Nat. Rev. Immunol. 4:595-602. - PubMed
-
- Blanchard, T. J., A. Alcami, P. Andrea, and G. L. Smith. 1998. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J. Gen. Virol. 79:1159-1167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

