Preclinical studies of a modified vaccinia virus Ankara-based HIV candidate vaccine: antigen presentation and antiviral effect
- PMID: 20219934
- PMCID: PMC2863849
- DOI: 10.1128/JVI.02329-09
Preclinical studies of a modified vaccinia virus Ankara-based HIV candidate vaccine: antigen presentation and antiviral effect
Abstract
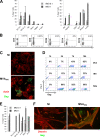
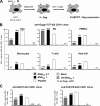
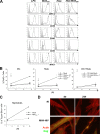
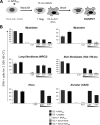
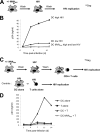
Poxvirus-based human immunodeficiency virus (HIV) vaccine candidates are currently under evaluation in preclinical and clinical trials. Modified vaccinia virus Ankara (MVA) vectors have excellent safety and immunogenicity records, but their behavior in human cell cultures remains only partly characterized. We studied here various virological and immunological aspects of the interactions of MVA-HIV, a vaccine candidate developed by the French National Agency for AIDS Research (ANRS), with primary human cells. We report that MVA-HIV infects and drives Gag expression in primary macrophages, dendritic cells (DCs), and epithelial and muscle cells. MVA-HIV-infected DCs matured, efficiently presented Gag, Pol, and Nef antigens, and activated HIV-specific cytotoxic T lymphocytes (CTLs). As expected with this type of vector, infection was cytopathic and led to DC apoptosis. Coculture of MVA-HIV-infected epithelial cells or myotubes with DCs promoted efficient Gag antigen major histocompatibility complex class I (MHC-I) cross-presentation without inducing direct infection and death of DCs. Antigen-presenting cells (APCs) infected with MVA-HIV also activated HIV-specific CD4(+) T cells. Moreover, exposure of DCs to MVA-HIV or to MVA-HIV-infected myotubes induced type I interferon (IFN) production and inhibited subsequent HIV replication and transfer to lymphocytes. Altogether, these results show that MVA-HIV promotes efficient MHC-I and MHC-II presentation of HIV antigens by APCs without facilitating HIV replication. Deciphering the immune responses to MVA in culture experiments will help in the design of innovative vaccine strategies.
Figures


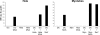
References
-
- Amara, R. R., F. Villinger, S. I. Staprans, J. D. Altman, D. C. Montefiori, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Different patterns of immune responses but similar control of a simian-human immunodeficiency virus 89.6P mucosal challenge by modified vaccinia virus Ankara (MVA) and DNA/MVA vaccines. J. Virol. 76:7625-7631. - PMC - PubMed
-
- Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385. - PubMed
-
- Bevan, M. J. 2004. Helping the CD8(+) T-cell response. Nat. Rev. Immunol. 4:595-602. - PubMed
-
- Blanchard, T. J., A. Alcami, P. Andrea, and G. L. Smith. 1998. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J. Gen. Virol. 79:1159-1167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

