Amino acid sensing by enteroendocrine STC-1 cells: role of the Na+-coupled neutral amino acid transporter 2
- PMID: 20219951
- PMCID: PMC2889636
- DOI: 10.1152/ajpcell.00518.2009
Amino acid sensing by enteroendocrine STC-1 cells: role of the Na+-coupled neutral amino acid transporter 2
Abstract
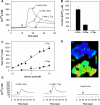
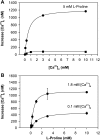
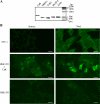
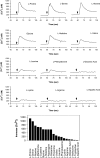
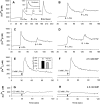
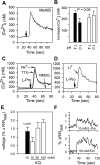
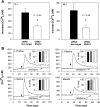
The results presented here show that STC-1 cells, a model of intestinal endocrine cells, respond to a broad range of amino acids, including l-proline, l-serine, l-alanine, l-methionine, l-glycine, l-histidine, and alpha-methyl-amino-isobutyric acid (MeAIB) with a rapid increase in the intracellular Ca(2+) concentration ([Ca(2+)](i)). We sought to identify the mechanism by which amino acids induce Ca(2+) signaling in these cells. Several lines of evidence suggest that amino acid transport through the Na(+)-coupled neutral amino acid transporter 2 (SNAT2) is a major mechanism by which amino acids induced Ca(2+) signaling in STC-1 cells: 1) the amino acid efficacy profile for inducing Ca(2+) signaling in STC-1 cells closely matches the amino acid specificity of SNAT2; 2) amino acid-induced Ca(2+) signaling in STC-1 cells was suppressed by removing Na(+) from the medium; 3) the nonmetabolized synthetic substrate of amino acid transport MeAIB produced a marked increase in [Ca(2+)](i); 4) transfection of small interfering RNA targeting SNAT2 produced a marked decrease in Ca(2+) signaling in response to l-proline in STC-1 cells; 5) amino acid-induced increase in [Ca(2+)](i) was associated with membrane depolarization and mediated by Ca(2+) influx, since it depended on extracellular Ca(2+); 6) the increase in [Ca(2+)](i) in response to l-proline, l-alanine, or MeAIB was abrogated by either nifedipine (1-10 muM) or nitrendipine (1 muM), which block L-type voltage-sensitive Ca(2+) channels. We hypothesize that the inward current of Na(+) associated with the function of SNAT2 leads to membrane depolarization and activation of voltage-sensitive Ca(2+) channels that mediate Ca(2+) influx, thereby leading to an increase in the [Ca(2+)](i) in enteroendocrine STC-1 cells.
Figures
References
-
- Ballantyne G. Peptide YY(1–36) and peptide YY(3–36). I. Distribution, release and actions. Obes Surg 16: 651–658, 2006. - PubMed
-
- Beach JM, McGahren ED, Xia J, Duling BR. Ratiometric measurement of endothelial depolarization in arterioles with a potential-sensitive dye. Am J Physiol Heart Circ Physiol 270: H2216–H2227, 1996 - PubMed
-
- Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 366: 575–580, 1993 - PubMed
-
- Buchan AMJ, Squires PE, Ring M, Meloche RM. Mechanism of action of the calcium-sensing receptor in human antral gastrin cells. Gastroenterology 120: 1128–1139, 2001 - PubMed
-
- Catterall WA, Striessnig J, Snutch TP, Perez-Reyes E. International Union of Pharmacology XL compendium of voltage-gated ion channels: calcium channels. Pharmacol Rev 55: 579–581, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

