Src-dependent TrkA transactivation is required for pituitary adenylate cyclase-activating polypeptide 38-mediated Rit activation and neuronal differentiation
- PMID: 20219970
- PMCID: PMC2861617
- DOI: 10.1091/mbc.e09-12-1033
Src-dependent TrkA transactivation is required for pituitary adenylate cyclase-activating polypeptide 38-mediated Rit activation and neuronal differentiation
Abstract
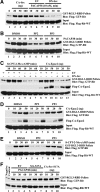
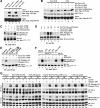
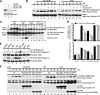
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a potent neuropeptide that possesses both neurotrophic and neurodevelopmental effects. Recently, the Rit GTPase was found to be activated by a novel Galpha/cAMP/exchange protein activated by cyclic AMP (Epac)-dependent signaling pathway and required for PACAP-dependent cAMP response element-binding protein activation and neuronal differentiation. However, Epac did not function as a Rit guanine nucleotide exchange factor (GEF), and the nature of the PACAP regulatory cascade remained unclear. Here, we show that PACAP-mediated Rit activation involves Src family kinase-dependent TrkA receptor transactivation. PACAP receptor (PACR1) stimulation triggered both G(i)alpha and G(s)alpha/cAMP/Epac regulatory cascades resulting in Src kinase activity, which in turn induced TrkA kinase tyrosine phosphorylation. Importantly, Src inhibition, or the lack of functional Trk receptors, was found to inhibit PACAP-mediated Rit activation, whereas constitutively active Src alone was sufficient to stimulate Rit-guanosine triphosphate levels. A single tyrosine (Y(499)) phosphorylation event was identified as critical to both PACAP-mediated transactivation and TrkA-dependent Rit activation. Accordingly, PACAP stimulation resulted in TrkA-dependent phosphorylation of both the Shc adaptor and son of sevenless (SOS)1/2 GEFs, and Rit activation was inhibited by RNA interference silencing of SOS1/2, implicating a TrkA/Shc/SOS signaling complex in Rit regulation. Together, these observations expand upon the nature of PACR1-mediated transactivation and identify TrkA-Rit signaling as a key contributor to PACAP-dependent neuronal differentiation.
Figures

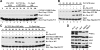


References
-
- Andres D. A., Rudolph J. L., Sengoku T., Shi G. X. Analysis of rit signaling and biological activity. Methods Enzymol. 2005;407:499–512. - PubMed
-
- Bernards A., Settleman J. GAP control: regulating the regulators of small GTPases. Trends Cell Biol. 2004;14:377–385. - PubMed
-
- Bos J. L. Epac: a new cAMP target and new avenues in cAMP research. Nat. Rev. Mol. Cell Biol. 2003;4:733–738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

