Stepwise assembly of dimeric F(1)F(o)-ATP synthase in mitochondria involves the small F(o)-subunits k and i
- PMID: 20219971
- PMCID: PMC2861609
- DOI: 10.1091/mbc.e09-12-1023
Stepwise assembly of dimeric F(1)F(o)-ATP synthase in mitochondria involves the small F(o)-subunits k and i
Abstract
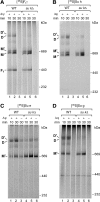
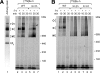
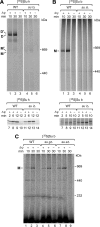
F(1)F(o)-ATP synthase is a key enzyme of oxidative phosphorylation that is localized in the inner membrane of mitochondria. It uses the energy stored in the proton gradient across the inner mitochondrial membrane to catalyze the synthesis of ATP from ADP and phosphate. Dimeric and higher oligomeric forms of ATP synthase have been observed in mitochondria from various organisms. Oligomerization of ATP synthase is critical for the morphology of the inner mitochondrial membrane because it supports the generation of tubular cristae membrane domains. Association of individual F(1)F(o)-ATP synthase complexes is mediated by the membrane-embedded F(o)-part. Several subunits were mapped to monomer-monomer-interfaces of yeast ATP synthase complexes, but only Su e (Atp21) and Su g (Atp20) have so far been identified as crucial for the formation of stable dimers. We show that two other small F(o)-components, Su k (Atp19) and Su i (Atp18) are involved in the stepwise assembly of F(1)F(o)-ATP synthase dimers and oligomers. We have identified an intermediate form of the ATP synthase dimer, which accumulates in the absence of Su i. Moreover, our data indicate that Su i facilitates the incorporation of newly synthesized subunits into ATP synthase complexes.
Figures






References
-
- Acín-Pérez R., Fernández-Silva P., Peleato M. L., Pérez-Martos A., Enriquez J. A. Respiratory active mitochondrial supercomplexes. Mol. Cell. 2008;32:529–539. - PubMed
-
- Allen R. D. Membrane tubulation and proton pumps. Protoplasma. 1995;189:1–8.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

