Glutathione peroxidase-1 modulates lipopolysaccharide-induced adhesion molecule expression in endothelial cells by altering CD14 expression
- PMID: 20219985
- PMCID: PMC2887263
- DOI: 10.1096/fj.09-147421
Glutathione peroxidase-1 modulates lipopolysaccharide-induced adhesion molecule expression in endothelial cells by altering CD14 expression
Abstract
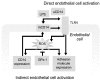
CD14 contributes to LPS signaling in leukocytes through formation of toll-like receptor 4/CD14 receptor complexes; however, a specific role for endogenous cell-surface CD14 in endothelial cells is unclear. We have found that suppression of glutathione peroxidase-1 (GPx-1) in human microvascular endothelial cells increases CD14 gene expression compared to untreated or siControl (siCtrl)-treated conditions. Following LPS treatment, GPx-1 deficiency augmented LPS-induced intracellular reactive oxygen species accumulation, CD14 expression, and intercellular adhesion molecule-1 (ICAM-1) mRNA and protein expression compared to LPS-treated control cells. GPx-1 deficiency also transiently augmented LPS-induced vascular cell adhesion molecule-1 (VCAM-1) expression. Adenoviral overexpression of GPx-1 significantly diminished LPS-mediated responses in adhesion molecule expression. Consistent with these findings, LPS responses were also greater in endothelial cells derived from GPx-1-knockout mice, whereas adhesion molecule expression was decreased in cells from GPx-1-overexpressing transgenic mice. Knockdown of CD14 attenuated LPS-mediated up-regulation of ICAM-1 and VCAM-1 mRNA and protein, and it mitigated the effects of GPx-1 deficiency on LPS-induced adhesion molecule expression. Taken together, these data suggest that GPx-1 modulates the endothelial cell response to LPS, in part, by altering CD14-mediated effects.
Figures


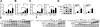

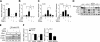

Similar articles
-
Glutathione peroxidase-1 deficiency augments proinflammatory cytokine-induced redox signaling and human endothelial cell activation.J Biol Chem. 2011 Oct 14;286(41):35407-35417. doi: 10.1074/jbc.M110.205708. Epub 2011 Aug 18. J Biol Chem. 2011. PMID: 21852236 Free PMC article.
-
Diclofenac inhibits endothelial cell adhesion molecule expression induced with lipopolysaccharide.Life Sci. 1996 May 24;58(26):2377-87. doi: 10.1016/0024-3205(96)00241-x. Life Sci. 1996. PMID: 8691982
-
Interleukin-4 and lipopolysaccharide synergize to induce vascular cell adhesion molecule-1 expression in human lung microvascular endothelial cells.Am J Respir Cell Mol Biol. 1998 May;18(5):620-30. doi: 10.1165/ajrcmb.18.5.3052. Am J Respir Cell Mol Biol. 1998. PMID: 9569232
-
Monocyte-endothelial cell interaction induces expression of adhesion molecules on human umbilical cord endothelial cells.Cardiovasc Res. 1996 Aug;32(2):422-9. doi: 10.1016/0008-6363(96)00085-5. Cardiovasc Res. 1996. PMID: 8796130
-
Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation.Circ Res. 1999 Jul 23;85(2):199-207. doi: 10.1161/01.res.85.2.199. Circ Res. 1999. PMID: 10417402
Cited by
-
NADPH Oxidase 3: Beyond the Inner Ear.Antioxidants (Basel). 2024 Feb 8;13(2):219. doi: 10.3390/antiox13020219. Antioxidants (Basel). 2024. PMID: 38397817 Free PMC article. Review.
-
Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities.Antioxid Redox Signal. 2011 Oct 1;15(7):1957-97. doi: 10.1089/ars.2010.3586. Epub 2011 Apr 10. Antioxid Redox Signal. 2011. PMID: 21087145 Free PMC article. Review.
-
Systems analysis of oxidant stress in the vasculature.IUBMB Life. 2013 Nov;65(11):911-20. doi: 10.1002/iub.1221. Epub 2013 Nov 7. IUBMB Life. 2013. PMID: 24265198 Free PMC article.
-
Oxidant stress regulatory genetic variation in recipients and donors contributes to risk of primary graft dysfunction after lung transplantation.J Thorac Cardiovasc Surg. 2015 Feb;149(2):596-602. doi: 10.1016/j.jtcvs.2014.09.077. Epub 2014 Sep 28. J Thorac Cardiovasc Surg. 2015. PMID: 25439478 Free PMC article.
-
The Intriguing Role of TLR Accessory Molecules in Cardiovascular Health and Disease.Front Cardiovasc Med. 2022 Feb 14;9:820962. doi: 10.3389/fcvm.2022.820962. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35237675 Free PMC article. Review.
References
-
- Cai H, Harrison D G. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. - PubMed
-
- Lapenna D, de Gioia S, Ciofani G, Mezzetti A, Ucchino S, Calafiore A M, Napolitano A M, Di Ilio C, Cuccurullo F. Glutathione-related antioxidant defenses in human atherosclerotic plaques. Circulation. 1998;97:1930–1934. - PubMed
-
- Raes M, Michiels C, Remacle J. Comparative study of the enzymatic defense systems against oxygen-derived free radicals: the key role of glutathione peroxidase. Free Radic Biol Med. 1987;3:3–7. - PubMed
-
- Ursini F, Maiorino M, Brigelius-Flohe R, Aumann K D, Roveri A, Schomburg D, Flohe L. Diversity of glutathione peroxidases. Methods Enzymol. 1995;252:38–53. - PubMed
-
- Crack P J, Taylor J M, Flentjar N J, de Haan J, Hertzog P, Iannello R C, Kola I. Increased infarct size and exacerbated apoptosis in the glutathione peroxidase-1 (Gpx-1) knockout mouse brain in response to ischemia/reperfusion injury. J Neurochem. 2001;78:1389–1399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

