Hedgehog signaling governs the development of otic sensory epithelium and its associated innervation in zebrafish
- PMID: 20219995
- PMCID: PMC6632237
- DOI: 10.1523/JNEUROSCI.5109-09.2010
Hedgehog signaling governs the development of otic sensory epithelium and its associated innervation in zebrafish
Abstract
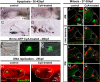
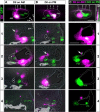
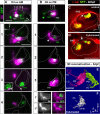
The inner ear is responsible for the perception of motion and sound in vertebrates. Its functional unit, the sensory patch, contains mechanosensory hair cells innervated by sensory neurons from the statoacoustic ganglion (SAG) that project to the corresponding nuclei in the brainstem. How hair cells develop at specific positions, and how otic neurons are sorted to specifically innervate each endorgan and to convey the extracted information to the hindbrain is not completely understood. In this work, we study the generation of macular sensory patches and investigate the role of Hedgehog (Hh) signaling in the production of their neurosensory elements. Using zebrafish transgenic lines to visualize the dynamics of hair cell and neuron production, we show that the development of the anterior and posterior maculae is asynchronic, suggesting they are independently regulated. Tracing experiments demonstrate the SAG is topologically organized in two different neuronal subpopulations, which are spatially segregated and innervate specifically each macula. Functional experiments identify the Hh pathway as crucial in coordinating the production of hair cells in the posterior macula, and the formation of its specific innervation. Finally, gene expression analyses suggest that Hh influences the balance between different SAG neuronal subpopulations. These results lead to a model in which Hh orients functionally the development of inner ear towards an auditory fate in all vertebrate species.
Figures



Similar articles
-
Fgf and Hh signalling act on a symmetrical pre-pattern to specify anterior and posterior identity in the zebrafish otic placode and vesicle.Development. 2011 Sep;138(18):3977-87. doi: 10.1242/dev.066639. Epub 2011 Aug 10. Development. 2011. PMID: 21831919 Free PMC article.
-
The transcription factor six1 inhibits neuronal and promotes hair cell fate in the developing zebrafish (Danio rerio) inner ear.J Neurosci. 2006 Oct 11;26(41):10438-51. doi: 10.1523/JNEUROSCI.1025-06.2006. J Neurosci. 2006. PMID: 17035528 Free PMC article.
-
Hedgehog signaling regulates sensory cell formation and auditory function in mice and humans.J Neurosci. 2008 Jul 16;28(29):7350-8. doi: 10.1523/JNEUROSCI.0312-08.2008. J Neurosci. 2008. PMID: 18632939 Free PMC article.
-
[Roles of the FGF signaling pathway in regulating inner ear development and hair cell regeneration].Yi Chuan. 2018 Jul 20;40(7):515-524. doi: 10.16288/j.yczz.17-407. Yi Chuan. 2018. PMID: 30021715 Review. Chinese.
-
Signaling regulating inner ear development: cell fate determination, patterning, morphogenesis, and defects.Congenit Anom (Kyoto). 2015 Feb;55(1):17-25. doi: 10.1111/cga.12072. Congenit Anom (Kyoto). 2015. PMID: 25040109 Review.
Cited by
-
Vestibular physiology and function in zebrafish.Front Cell Dev Biol. 2023 Apr 18;11:1172933. doi: 10.3389/fcell.2023.1172933. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37143895 Free PMC article. Review.
-
Connecting the ear to the brain: Molecular mechanisms of auditory circuit assembly.Prog Neurobiol. 2011 Apr;93(4):488-508. doi: 10.1016/j.pneurobio.2011.01.004. Epub 2011 Jan 11. Prog Neurobiol. 2011. PMID: 21232575 Free PMC article. Review.
-
The transcription factor, Nuclear factor, erythroid 2 (Nfe2), is a regulator of the oxidative stress response during Danio rerio development.Aquat Toxicol. 2016 Nov;180:141-154. doi: 10.1016/j.aquatox.2016.09.019. Epub 2016 Oct 1. Aquat Toxicol. 2016. PMID: 27716579 Free PMC article.
-
A spatial and temporal gradient of Fgf differentially regulates distinct stages of neural development in the zebrafish inner ear.PLoS Genet. 2012;8(11):e1003068. doi: 10.1371/journal.pgen.1003068. Epub 2012 Nov 15. PLoS Genet. 2012. PMID: 23166517 Free PMC article.
-
Anteroposterior patterning of the zebrafish ear through Fgf- and Hh-dependent regulation of hmx3a expression.PLoS Genet. 2019 Apr 25;15(4):e1008051. doi: 10.1371/journal.pgen.1008051. eCollection 2019 Apr. PLoS Genet. 2019. PMID: 31022185 Free PMC article.
References
-
- Andermann P, Ungos J, Raible DW. Neurogenin1 defines zebrafish cranial sensory ganglia precursors. Dev Biol. 2002;251:45–58. - PubMed
-
- Bell D, Streit A, Gorospe I, Varela-Nieto I, Alsina B, Giraldez F. Spatial and temporal segregation of auditory and vestibular neurons in the otic placode. Dev Biol. 2008;322:109–120. - PubMed
-
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. - PubMed
-
- Bever MM, Fekete DM. Ventromedial focus of cell death is absent during development of Xenopus and zebrafish inner ears. J Neurocytol. 1999;28:781–793. - PubMed
-
- Bok J, Bronner-Fraser M, Wu DK. Role of the hindbrain in dorsoventral but not anteroposterior axial specification of the inner ear. Development. 2005;132:2115–2124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
