GSK-3 beta inhibits presynaptic vesicle exocytosis by phosphorylating P/Q-type calcium channel and interrupting SNARE complex formation
- PMID: 20219996
- PMCID: PMC6632254
- DOI: 10.1523/JNEUROSCI.5223-09.2010
GSK-3 beta inhibits presynaptic vesicle exocytosis by phosphorylating P/Q-type calcium channel and interrupting SNARE complex formation
Abstract
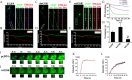
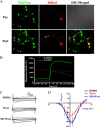
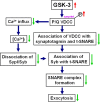
Glycogen synthase kinase-3 (GSK-3), a Ser/Thr protein kinase abundantly expressed in neurons, plays diverse functions in physiological and neurodegenerative conditions. Our recent study shows that upregulation of GSK-3 suppresses long-term potentiation and presynaptic release of glutamate; however, the underlying mechanism is elusive. Here, we show that activation of GSK-3beta retards the synaptic vesicle exocytosis in response to membrane depolarization. Using calcium imaging, whole-cell patch-clamp, as well as specific Ca(2+) channel inhibitors, we demonstrate that GSK-3beta phosphorylates the intracellular loop-connecting domains II and III (L(II-III)) of P/Q-type Ca(2+) channels, which leads to a decrease of intracellular Ca(2+) rise through the P/Q-type voltage-dependent calcium channel. To further illustrate the mechanisms of GSK-3beta's action, we show that activation of GSK-3beta interferes with the formation of the soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP) receptor (SNARE) complex through: (1) weakening the association of synaptobrevin with SNAP25 and syntaxin; (2) reducing the interactions among the phosphorylated L(II-III) and synaptotagmin, SNAP25, and syntaxin; and (3) inhibiting dissociation of synaptobrevin from synaptophysin I. These results indicate that GSK-3beta negatively regulates synaptic vesicle fusion events via interfering with Ca(2+)-dependent SNARE complex formation.
Figures



Similar articles
-
Cdk5/p35 regulates neurotransmitter release through phosphorylation and downregulation of P/Q-type voltage-dependent calcium channel activity.J Neurosci. 2002 Apr 1;22(7):2590-7. doi: 10.1523/JNEUROSCI.22-07-02590.2002. J Neurosci. 2002. PMID: 11923424 Free PMC article.
-
Efferent function of vestibular afferent endings? Similar localization of N-type calcium channels, synaptic vesicle and synaptic membrane-associated proteins.Neuroscience. 2000;98(2):377-84. doi: 10.1016/s0306-4522(00)00119-6. Neuroscience. 2000. PMID: 10854771
-
Ca2+ -independent and voltage-dependent exocytosis in mouse chromaffin cells.Acta Physiol (Oxf). 2020 Apr;228(4):e13417. doi: 10.1111/apha.13417. Epub 2019 Dec 11. Acta Physiol (Oxf). 2020. PMID: 31769918
-
Interactions between proteins implicated in exocytosis and voltage-gated calcium channels.Philos Trans R Soc Lond B Biol Sci. 1999 Feb 28;354(1381):289-97. doi: 10.1098/rstb.1999.0380. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10212477 Free PMC article. Review.
-
Diversity of presynaptic calcium channels displaying different synaptic properties.Rev Neurosci. 2012 Feb 29;23(2):179-90. doi: 10.1515/revneuro-2011-0070. Rev Neurosci. 2012. PMID: 22499676 Review.
Cited by
-
Lentiviral silencing of GSK-3β in adult dentate gyrus impairs contextual fear memory and synaptic plasticity.Front Behav Neurosci. 2015 Jun 23;9:158. doi: 10.3389/fnbeh.2015.00158. eCollection 2015. Front Behav Neurosci. 2015. PMID: 26157370 Free PMC article.
-
Inhibition of glycogen synthase kinase-3β prevents remifentanil-induced hyperalgesia via regulating the expression and function of spinal N-methyl-D-aspartate receptors in vivo and vitro.PLoS One. 2013 Oct 16;8(10):e77790. doi: 10.1371/journal.pone.0077790. eCollection 2013. PLoS One. 2013. PMID: 24147079 Free PMC article.
-
Endoplasmic reticulum stress induces spatial memory deficits by activating GSK-3.J Cell Mol Med. 2018 Jul;22(7):3489-3502. doi: 10.1111/jcmm.13626. Epub 2018 Apr 19. J Cell Mol Med. 2018. PMID: 29675957 Free PMC article.
-
The MT2 receptor stimulates axonogenesis and enhances synaptic transmission by activating Akt signaling.Cell Death Differ. 2015 Apr;22(4):583-96. doi: 10.1038/cdd.2014.195. Epub 2014 Dec 12. Cell Death Differ. 2015. PMID: 25501601 Free PMC article.
-
Herpes Simplex Virus type-1 infection induces synaptic dysfunction in cultured cortical neurons via GSK-3 activation and intraneuronal amyloid-β protein accumulation.Sci Rep. 2015 Oct 21;5:15444. doi: 10.1038/srep15444. Sci Rep. 2015. PMID: 26487282 Free PMC article.
References
-
- Bacci A, Coco S, Pravettoni E, Schenk U, Armano S, Frassoni C, Verderio C, De Camilli P, Matteoli M. Chronic blockade of glutamate receptors enhances presynaptic release and downregulates the interaction between synaptophysin-synaptobrevin-vesicle-associated membrane protein 2. J Neurosci. 2001;21:6588–6596. - PMC - PubMed
-
- Becherer U, Moser T, Stühmer W, Oheim M. Calcium regulates exocytosis at the level of single vesicles. Nat Neurosci. 2003;6:846–853. - PubMed
-
- Bourinet E, Soong TW, Sutton K, Slaymaker S, Mathews E, Monteil A, Zamponi GW, Nargeot J, Snutch TP. Splicing of alpha 1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nat Neurosci. 1999;2:407–415. - PubMed
-
- Carnes CA, Janssen PM, Ruehr ML, Nakayama H, Nakayama T, Haase H, Bauer JA, Chung MK, Fearon IM, Gillinov AM, Hamlin RL, Van Wagoner DR. Atrial glutathione content, calcium current, and contractility. J Biol Chem. 2007;282:28063–28073. - PubMed
-
- Catterall WA. Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release. Cell Calcium. 1998;24:307–323. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
