Developmental tuning and decay in senescence of oscillations linking the corticospinal system
- PMID: 20220000
- PMCID: PMC6632255
- DOI: 10.1523/JNEUROSCI.5621-09.2010
Developmental tuning and decay in senescence of oscillations linking the corticospinal system
Abstract
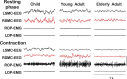
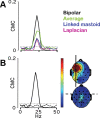
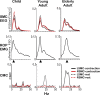
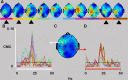
There is increasing evidence of the importance of synchronous activity within the corticospinal system for motor control. We compared oscillatory activity in the primary sensorimotor cortex [EEG of sensorimotor cortex (SMC-EEG)] and a motor neuronal pool [surface electromyogram of opponens pollicis (OP-EMG)], and their coherence in children (4-12 years of age), young adults (20-35 years of age), and elderly adults (>55 years of age). The ratio between lower (2-13 Hz) and higher (14-32 Hz) frequencies in both SMC-EEG and OP-EMG decreased with age, correlating inversely with motor performance. There was evidence for larger, more distributed cortical networks in the children and elderly compared with young adults. Corticomuscular coherence (CMC) was present in all age groups and shifted between frontal and parietal cortical areas. In children, CMC was smaller and less stationary in amplitude and frequency than in adults. Young adults had single peaks of CMC clustered near the modal frequency (23 Hz); multiple peaks with a broad spread of frequencies occurred in children and the elderly; the further the frequency of the maximum peak CMC was from 23 Hz, the poorer the performance. CMC amplitude was inversely related to performance in young adults but was not modulated in relation to performance in children and the elderly. We propose that progressive fine-tuning of the frequency coding and stabilization of the dynamic properties within and between corticospinal networks occurs during adolescence, refining the capacity for efficient dynamic communication in adulthood. In old age, blurring of the tuning between networks and breakdown in their integration occurs and is likely to contribute to a decrement in motor control.
Figures
Similar articles
-
Dynamics of corticospinal motor control during overground and treadmill walking in humans.J Neurophysiol. 2018 Sep 1;120(3):1017-1031. doi: 10.1152/jn.00613.2017. Epub 2018 May 30. J Neurophysiol. 2018. PMID: 29847229
-
Corticomuscular synchronization with small and large dynamic force output.BMC Neurosci. 2007 Nov 27;8:101. doi: 10.1186/1471-2202-8-101. BMC Neurosci. 2007. PMID: 18042289 Free PMC article.
-
It is not all about phase: amplitude dynamics in corticomuscular interactions.Neuroimage. 2013 Jan 1;64:496-504. doi: 10.1016/j.neuroimage.2012.08.069. Epub 2012 Aug 30. Neuroimage. 2013. PMID: 22960151
-
A critical period of corticomuscular and EMG-EMG coherence detection in healthy infants aged 9-25 weeks.J Physiol. 2017 Apr 15;595(8):2699-2713. doi: 10.1113/JP273090. Epub 2017 Feb 15. J Physiol. 2017. PMID: 28004392 Free PMC article.
-
The myth of the 'unaffected' side after unilateral stroke: is reorganisation of the non-infarcted corticospinal system to re-establish balance the price for recovery?Exp Neurol. 2012 Dec;238(2):168-75. doi: 10.1016/j.expneurol.2012.08.031. Epub 2012 Sep 7. Exp Neurol. 2012. PMID: 22981842 Free PMC article.
Cited by
-
Greater intermanual transfer in the elderly suggests age-related bilateral motor cortex activation is compensatory.J Mot Behav. 2015;47(1):47-55. doi: 10.1080/00222895.2014.981501. J Mot Behav. 2015. PMID: 25575222 Free PMC article.
-
On the Homology of the Dominant and Non-Dominant Corticospinal Tracts: A Novel Neurophysiological Assessment.Brain Sci. 2023 Feb 7;13(2):278. doi: 10.3390/brainsci13020278. Brain Sci. 2023. PMID: 36831821 Free PMC article.
-
Dynamic cortical participation during bilateral, cyclical ankle movements: effects of aging.Sci Rep. 2017 Mar 16;7:44658. doi: 10.1038/srep44658. Sci Rep. 2017. PMID: 28300175 Free PMC article. Clinical Trial.
-
Cerebral palsy.Nat Rev Dis Primers. 2016 Jan 7;2:15082. doi: 10.1038/nrdp.2015.82. Nat Rev Dis Primers. 2016. PMID: 27188686 Free PMC article. Review.
-
Functional balance at rest of hemispheric homologs assessed via normalized compression distance.Front Neurosci. 2024 Jan 25;17:1261701. doi: 10.3389/fnins.2023.1261701. eCollection 2023. Front Neurosci. 2024. PMID: 38333603 Free PMC article.
References
-
- Anderson B, Rutledge V. Age and hemisphere effects on dendritic structure. Brain. 1996;119:1983–1990. - PubMed
-
- Andrew C, Pfurtscheller G. Dependence of coherence measurements on EEG derivation type. Med Biol Eng Comput. 1996;34:232–238. - PubMed
-
- Baker SN, Pinches EM, Lemon RN. Synchronization in monkey motor cortex during a precision grip task. II. Effect of oscillatory activity on corticospinal output. J Neurophysiol. 2003;89:1941–1953. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
