Changes in simple spike activity of some Purkinje cells in the oculomotor vermis during saccade adaptation are appropriate to participate in motor learning
- PMID: 20220005
- PMCID: PMC2864307
- DOI: 10.1523/JNEUROSCI.4953-09.2010
Changes in simple spike activity of some Purkinje cells in the oculomotor vermis during saccade adaptation are appropriate to participate in motor learning
Abstract
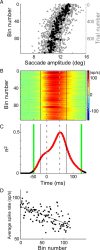
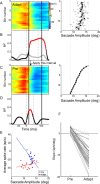
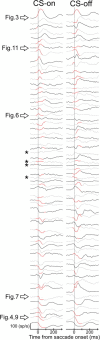
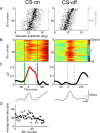
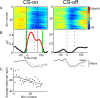
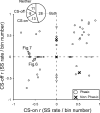
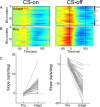
Adaptation of saccadic eye movements provides an excellent motor learning model to study theories of neuronal plasticity. When primates make saccades to a jumping target, a backward step of the target during the saccade can make it appear to overshoot. If this deception continues for many trials, saccades gradually decrease in amplitude to go directly to the back-stepped target location. We used this adaptation paradigm to evaluate the Marr-Albus hypothesis that such motor learning occurs at the Purkinje (P)-cell of the cerebellum. We recorded the activity of identified P-cells in the oculomotor vermis, lobules VIc and VII. After documenting the on and off error directions of the complex spike activity of a P-cell, we determined whether its saccade-related simple spike (SS) activity changed during saccade adaptation in those two directions. Before adaptation, 57 of 61 P-cells exhibited a clear burst, pause, or a combination of both for saccades in one or both directions. Sixty-two percent of all cells, including two of the four initially unresponsive ones, behaved differently for saccades whose size changed because of adaptation than for saccades of similar sizes gathered before adaptation. In at least 42% of these, the changes were appropriate to decrease saccade amplitude based on our current knowledge of cerebellum and brainstem saccade circuitry. Changes in activity during adaptation were not compensating for the potential fatigue associated with performing many saccades. Therefore, many P-cells in the oculomotor vermis exhibit changes in SS activity specific to adapted saccades and therefore appropriate to induce adaptation.
Figures


Similar articles
-
Discharge properties of Purkinje cells in the oculomotor vermis during visually guided saccades in the macaque monkey.J Neurophysiol. 1995 Nov;74(5):1828-40. doi: 10.1152/jn.1995.74.5.1828. J Neurophysiol. 1995. PMID: 8592177
-
Complex spike activity of purkinje cells in the oculomotor vermis during behavioral adaptation of monkey saccades.J Neurosci. 2006 Jul 19;26(29):7741-55. doi: 10.1523/JNEUROSCI.4658-05.2006. J Neurosci. 2006. PMID: 16855102 Free PMC article.
-
How cerebellar motor learning keeps saccades accurate.J Neurophysiol. 2019 Jun 1;121(6):2153-2162. doi: 10.1152/jn.00781.2018. Epub 2019 Apr 17. J Neurophysiol. 2019. PMID: 30995136 Free PMC article. Review.
-
Complex spike activity in the oculomotor vermis of the cerebellum: a vectorial error signal for saccade motor learning?J Neurophysiol. 2008 Oct;100(4):1949-66. doi: 10.1152/jn.90526.2008. Epub 2008 Jul 23. J Neurophysiol. 2008. PMID: 18650308 Free PMC article.
-
Cerebellar control of saccadic eye movements: its neural mechanisms and pathways.Jpn J Physiol. 1991;41(3):351-68. doi: 10.2170/jjphysiol.41.351. Jpn J Physiol. 1991. PMID: 1960885 Review.
Cited by
-
Short-term saccadic adaptation in the macaque monkey: a binocular mechanism.J Neurophysiol. 2013 Jan;109(2):518-45. doi: 10.1152/jn.01013.2011. Epub 2012 Oct 17. J Neurophysiol. 2013. PMID: 23076111 Free PMC article.
-
Circuit mechanisms underlying motor memory formation in the cerebellum.Neuron. 2015 Apr 22;86(2):529-40. doi: 10.1016/j.neuron.2015.03.010. Epub 2015 Apr 2. Neuron. 2015. PMID: 25843404 Free PMC article.
-
Impaired Motor Learning in a Disorder of the Inferior Olive: Is the Cerebellum Confused?Cerebellum. 2017 Feb;16(1):158-167. doi: 10.1007/s12311-016-0785-x. Cerebellum. 2017. PMID: 27165043 Free PMC article.
-
P-sort: an open-source software for cerebellar neurophysiology.J Neurophysiol. 2021 Oct 1;126(4):1055-1075. doi: 10.1152/jn.00172.2021. Epub 2021 Aug 25. J Neurophysiol. 2021. PMID: 34432996 Free PMC article.
-
Behavior of the oculomotor vermis for five different types of saccade.J Neurophysiol. 2010 Dec;104(6):3667-76. doi: 10.1152/jn.00558.2010. Epub 2010 Oct 20. J Neurophysiol. 2010. PMID: 20962069 Free PMC article.
References
-
- Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61.
-
- Cherny E, Galloway E, Kaneko CRS, Noto CT, Robinson FR. Bilateral projection from the oculomotor vermis to the caudal fastigial nucleus in monkey. Soc Neurosci Abstr. 2005;31:755–3.
-
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966;21:1068–1070. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
