Bradykinin and nerve growth factor play pivotal roles in muscular mechanical hyperalgesia after exercise (delayed-onset muscle soreness)
- PMID: 20220009
- PMCID: PMC6632252
- DOI: 10.1523/JNEUROSCI.3803-09.2010
Bradykinin and nerve growth factor play pivotal roles in muscular mechanical hyperalgesia after exercise (delayed-onset muscle soreness)
Abstract
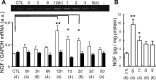
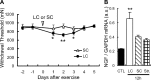
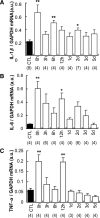
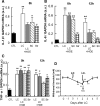
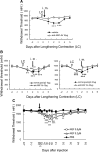
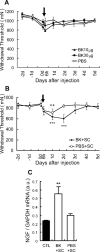
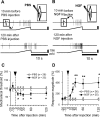
Unaccustomed strenuous exercise that includes lengthening contraction (LC) often causes delayed-onset muscle soreness (DOMS), a kind of muscular mechanical hyperalgesia. The substances that induce this phenomenon are largely unknown. Peculiarly, DOMS is not perceived during and shortly after exercise, but rather is first perceived after approximately 1 d. Using B(2) bradykinin receptor antagonist HOE 140, we show here that bradykinin released during exercise plays a pivotal role in triggering the process that leads to muscular mechanical hyperalgesia. HOE 140 completely suppressed the development of muscular mechanical hyperalgesia when injected before LC, but when injected 2 d after LC failed to reverse mechanical hyperalgesia that had already developed. B(1) antagonist was ineffective, regardless of the timing of its injection. Upregulation of nerve growth factor (NGF) mRNA and protein occurred in exercised muscle over a comparable time course (12 h to 2 d after LC) for muscle mechanical hyperalgesia. Antibodies to NGF injected intramuscularly 2 d after exercise reversed muscle mechanical hyperalgesia. HOE 140 inhibited the upregulation of NGF. In contrast, shortening contraction or stretching induced neither mechanical hyperalgesia nor NGF upregulation. Bradykinin together with shortening contraction, but not bradykinin alone, reproduced lasting mechanical hyperalgesia. We also showed that rat NGF sensitized thin-fiber afferents to mechanical stimulation in the periphery after 10-20 min. Thus, NGF upregulation through activation of B(2) bradykinin receptors is essential (though not satisfactory) to mechanical hyperalgesia after exercise. The present observations explain why DOMS occurs with a delay, and why lengthening contraction but not shortening contraction induces DOMS.
Figures

Similar articles
-
Absence of mechanical hyperalgesia after exercise (delayed onset muscle soreness) in neonatally capsaicin-treated rats.Neurosci Res. 2012 May;73(1):56-60. doi: 10.1016/j.neures.2012.02.005. Epub 2012 Feb 21. Neurosci Res. 2012. PMID: 22381959
-
Upregulated glial cell line-derived neurotrophic factor through cyclooxygenase-2 activation in the muscle is required for mechanical hyperalgesia after exercise in rats.J Physiol. 2013 Jun 15;591(12):3035-48. doi: 10.1113/jphysiol.2012.249235. Epub 2013 Apr 15. J Physiol. 2013. PMID: 23587883 Free PMC article.
-
Decreased nerve growth factor upregulation is a mechanism for reduced mechanical hyperalgesia after the second bout of exercise in rats.Scand J Med Sci Sports. 2013 Mar;23(2):e96-101. doi: 10.1111/sms.12013. Epub 2012 Nov 8. Scand J Med Sci Sports. 2013. PMID: 23134144
-
Delayed onset muscle soreness: Involvement of neurotrophic factors.J Physiol Sci. 2016 Jan;66(1):43-52. doi: 10.1007/s12576-015-0397-0. J Physiol Sci. 2016. PMID: 26467448 Free PMC article. Review.
-
Neurochemical mechanism of muscular pain: Insight from the study on delayed onset muscle soreness.J Physiol Sci. 2024 Jan 24;74(1):4. doi: 10.1186/s12576-023-00896-y. J Physiol Sci. 2024. PMID: 38267849 Free PMC article. Review.
Cited by
-
Leucine-enriched essential amino acids attenuate muscle soreness and improve muscle protein synthesis after eccentric contractions in rats.Amino Acids. 2015 Jun;47(6):1193-201. doi: 10.1007/s00726-015-1946-9. Epub 2015 Mar 14. Amino Acids. 2015. PMID: 25772815 Free PMC article.
-
Neurobiology of fibromyalgia and chronic widespread pain.Neuroscience. 2016 Dec 3;338:114-129. doi: 10.1016/j.neuroscience.2016.06.006. Epub 2016 Jun 9. Neuroscience. 2016. PMID: 27291641 Free PMC article. Review.
-
A review of nutritional intervention on delayed onset muscle soreness. Part I.J Exerc Rehabil. 2014 Dec 31;10(6):349-56. doi: 10.12965/jer.140179. eCollection 2014 Dec. J Exerc Rehabil. 2014. PMID: 25610818 Free PMC article. Review.
-
Brief research report: Repurposing pentoxifylline to treat intense acute swimming-Induced delayed-onset muscle soreness in mice: Targeting peripheral and spinal cord nociceptive mechanisms.Front Pharmacol. 2023 Jan 10;13:950314. doi: 10.3389/fphar.2022.950314. eCollection 2022. Front Pharmacol. 2023. PMID: 36703752 Free PMC article.
-
Spinal Mobilization Prevents NGF-Induced Trunk Mechanical Hyperalgesia and Attenuates Expression of CGRP.Front Neurosci. 2020 Apr 30;14:385. doi: 10.3389/fnins.2020.00385. eCollection 2020. Front Neurosci. 2020. PMID: 32425750 Free PMC article.
References
-
- Amano T, Yamakuni T, Okabe N, Sakimura K, Takahashi Y. Production of nerve growth-factor in rat skeletal-muscle. Neurosci Lett. 1991;132:5–7. - PubMed
-
- Armstrong RB. Mechanisms of exercise-induced delayed onset muscular soreness. Med Sci Sports Exerc. 1984;16:529–538. - PubMed
-
- Armstrong RB, Ogilvie RW, Schwane JA. Eccentric exercise-induced injury to rat skeletal-muscle. J Appl Physiol. 1983;54:80–93. - PubMed
-
- Bao G, Qadri F, Stauss B, Stauss H, Gohlke P, Unger T. HOE-140, a new highly potent and long-acting bradykinin antagonist in conscious rats. Eur J Pharmacol. 1991;200:179–182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
