Evaluation of the (R)VAD+C regimen for the treatment of newly diagnosed mantle cell lymphoma. Combined results of two prospective phase II trials from the French GOELAMS group
- PMID: 20220059
- PMCID: PMC2913084
- DOI: 10.3324/haematol.2009.011759
Evaluation of the (R)VAD+C regimen for the treatment of newly diagnosed mantle cell lymphoma. Combined results of two prospective phase II trials from the French GOELAMS group
Abstract
Background There is currently no international consensus for first-line treatment (prior to autologous stem cell transplantation) in mantle cell lymphoma patients. Here, we investigated the efficacy and tolerance of VAD associated with chlorambucil (VAD+C) and rituximab or not before autologous stem cell transplantation.
Design and methods: Between 1996 and 2005, 113 previously untreated mantle cell lymphoma patients were enrolled in two consecutive prospective phase II studies. Responses and response factors to the (R)VAD+C regimen were evaluated. The survival prognostic value of the MIPI score and Ki67 were also analyzed.
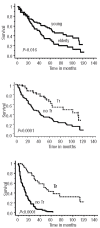
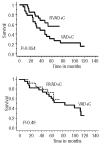
Results: The induction phase of 4 courses of (R)VAD+C showed very low hematologic and extra-hematologic toxicity (grade 3-4 thrombopenia and neutropenia, 9% and 2.7%, respectively and grade 3-4 extra-hematologic toxicities, 1.6%). Overall and complete response rates were 73% and 46%, respectively, and rose to 83% and 51% for the 70% of patients with less than two independent response factors (LDH, B symptoms and lymphocytosis). At the end of treatment, 65% of patients were in complete remission. Progression free and overall survival were significantly better in the transplanted population. The MIPI score was confirmed as a predictor of survival. Ki67, serum LDH, Performance Status (PS) and B symptoms were identified as independent prognostic factors of survival. A prognostic scoring system could stratify patients into three risk groups with markedly different median overall survival of 112, 44 and 11 months, respectively. Conclusions The (R)VAD+C is an effective regimen with very low toxicity. In addition to the MIPI score, Ki67 expression provides additional independent prognostic information for the prediction of overall survival (ClinicalTrials.gov Identifier: NCT00285389).
Figures


Comment in
-
Front-line treatment of mantle cell lymphoma.Haematologica. 2010 Aug;95(8):1241-3. doi: 10.3324/haematol.2010.025627. Haematologica. 2010. PMID: 20675744 Free PMC article. No abstract available.
References
-
- Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. The World Health Organization classification of neoplasms of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting--Airlie House, Virginia, November, 1997. Hematol J. 2000;1(1):53–66. - PubMed
-
- Herrmann A, Hoster E, Zwingers T, Brittinger G, Engelhard M, Meusers P, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27(4):511–8. - PubMed
-
- Bernard M, Gressin R, Lefrere F, Drenou B, Branger B, Caulet-Maugendre S, et al. Blastic variant of mantle cell lymphoma: a rare but highly aggressive subtype. Leukemia. 2001;15(11):1785–91. - PubMed
-
- Meusers P, Engelhard M, Bartels H, Binder T, Fulle HH, Gorg K, et al. Multicentre randomized therapeutic trial for advanced centrocytic lymphoma: anthracycline does not improve the prognosis. Hematol Oncol. 1989;7(5):365–80. - PubMed
-
- Zucca E, Roggero E, Pinotti G, Pedrinis E, Cappella C, Venco A, et al. Patterns of survival in mantle cell lymphoma. Ann Oncol. 1995;6(3):257–62. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

