Brief sounds evoke prolonged responses in anesthetized ferret auditory cortex
- PMID: 20220077
- PMCID: PMC2867571
- DOI: 10.1152/jn.00730.2009
Brief sounds evoke prolonged responses in anesthetized ferret auditory cortex
Abstract
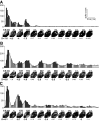
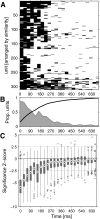
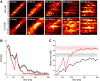
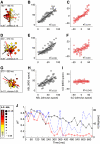
Neurons in the auditory cortex of anesthetized animals are generally considered to generate phasic responses to simple stimuli such as tones or noise bursts. In this paper, we show that under ketamine/medetomidine anesthesia, neurons in ferret auditory cortex usually exhibit complex sustained responses. We presented 100-ms broad-band noise bursts at a range of interaural level differences (ILDs) and average binaural levels (ABLs), and used extracellular electrodes to monitor evoked activity over 700 ms poststimulus onset. We estimated the degree of randomness (noise) in the response functions of individual neurons over poststimulus time; we found that neural activity was significantly modulated by sound for up to approximately 500 ms following stimulus offset. Pooling data from all neurons, we found that spiking activity carries significant information about stimulus identity over this same time period. However, information about ILD decayed much more quickly over time compared with information about ABL. In addition, ILD and ABL are coded independently by the neural population even though this is not the case at individual neurons. Though most neurons responded more strongly to ILDs corresponding to the opposite side of space, as a population, they were equally informative about both contra- and ipsilateral stimuli.
Figures


Similar articles
-
Binaural processing of sound pressure level in cat primary auditory cortex: evidence for a representation based on absolute levels rather than interaural level differences.J Neurophysiol. 1993 Feb;69(2):449-61. doi: 10.1152/jn.1993.69.2.449. J Neurophysiol. 1993. PMID: 8459277
-
Influence of callosal activity on units in the auditory cortex of ferret (Mustela putorius).J Neurophysiol. 1994 May;71(5):1740-51. doi: 10.1152/jn.1994.71.5.1740. J Neurophysiol. 1994. PMID: 8064345
-
Neuronal interaural level difference response shifts are level-dependent in the rat auditory cortex.J Neurophysiol. 2014 Mar;111(5):930-8. doi: 10.1152/jn.00648.2013. Epub 2013 Dec 11. J Neurophysiol. 2014. PMID: 24335208 Free PMC article.
-
Binaural interaction revisited in the cat primary auditory cortex.J Neurophysiol. 2004 Jan;91(1):101-17. doi: 10.1152/jn.00166.2003. Epub 2003 Sep 24. J Neurophysiol. 2004. PMID: 14507982
-
Visual influences on ferret auditory cortex.Hear Res. 2009 Dec;258(1-2):55-63. doi: 10.1016/j.heares.2009.06.017. Epub 2009 Jul 10. Hear Res. 2009. PMID: 19595754 Free PMC article. Review.
Cited by
-
Auditory cortical tuning to band-pass noise in primate A1 and CM: a comparison to pure tones.Neurosci Res. 2011 Aug;70(4):401-7. doi: 10.1016/j.neures.2011.04.003. Epub 2011 Apr 21. Neurosci Res. 2011. PMID: 21540062 Free PMC article.
-
Multidimensional receptive field processing by cat primary auditory cortical neurons.Neuroscience. 2017 Sep 17;359:130-141. doi: 10.1016/j.neuroscience.2017.07.003. Epub 2017 Jul 8. Neuroscience. 2017. PMID: 28694174 Free PMC article.
-
A novel coding mechanism for social vocalizations in the lateral amygdala.J Neurophysiol. 2012 Feb;107(4):1047-57. doi: 10.1152/jn.00422.2011. Epub 2011 Nov 16. J Neurophysiol. 2012. PMID: 22090463 Free PMC article.
-
Multisensory training improves auditory spatial processing following bilateral cochlear implantation.J Neurosci. 2014 Aug 13;34(33):11119-30. doi: 10.1523/JNEUROSCI.4767-13.2014. J Neurosci. 2014. PMID: 25122908 Free PMC article.
-
Multiplexed and robust representations of sound features in auditory cortex.J Neurosci. 2011 Oct 12;31(41):14565-76. doi: 10.1523/JNEUROSCI.2074-11.2011. J Neurosci. 2011. PMID: 21994373 Free PMC article.
References
-
- Anderson J, Lampl I, Reichova I, Carandini M, Ferster D. Stimulus dependence of two-state fluctuations of membrane potential in cat visual cortex. Nat Neurosci 3: 617–621, 2000. - PubMed
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 273: 1868–1871, 1996. - PubMed
-
- Bartlett EL, Wang X. Long-lasting modulation by stimulus context in primate auditory cortex. J Neurophysiol 94: 83–104, 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

