Retinohypothalamic tract synapses in the rat suprachiasmatic nucleus demonstrate short-term synaptic plasticity
- PMID: 20220078
- PMCID: PMC2867567
- DOI: 10.1152/jn.00695.2009
Retinohypothalamic tract synapses in the rat suprachiasmatic nucleus demonstrate short-term synaptic plasticity
Abstract
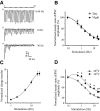
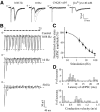
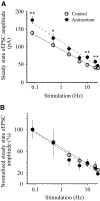
The master circadian pacemaker located in the suprachiasmatic nucleus (SCN) is entrained by light intensity-dependent signals transmitted via the retinohypothalamic tract (RHT). Short-term plasticity at glutamatergic RHT-SCN synapses was studied using stimulus frequencies that simulated the firing of light sensitive retinal ganglion cells. The evoked excitatory postsynaptic current (eEPSC) was recorded from SCN neurons located in hypothalamic brain slices. The eEPSC amplitude was stable during 0.08 Hz stimulation and exhibited frequency-dependent short-term synaptic depression (SD) during 0.5 to 100 Hz stimulus trains in 95 of 99 (96%) recorded neurons. During SD the steady-state eEPSC amplitude decreased, whereas the cumulative charge transfer increased in a frequency-dependent manner and saturated at 20 Hz. SD was similar during subjective day and night and decreased with increasing temperature. Paired-pulse stimulation (PPS) and voltage-dependent Ca(2+) channel (VDCC) blockers were used to characterize a presynaptic release mechanism. Facilitation was present in 30% and depression in 70% of studied neurons during PPS. Synaptic transmission was reduced by blocking both N- and P/Q-type presynaptic VDCCs, but only the N-type channel blocker significantly relieved SD. Aniracetam inhibited AMPA receptor desensitization but did not alter SD. Thus we concluded that SD is the principal form of short-term plasticity at RHT synapses, which presynaptically and frequency-dependently attenuates light-induced glutamatergic RHT synaptic transmission protecting SCN neurons against excessive excitation.
Figures


Similar articles
-
GABAB receptor-mediated frequency-dependent and circadian changes in synaptic plasticity modulate retinal input to the suprachiasmatic nucleus.J Physiol. 2013 May 15;591(10):2475-90. doi: 10.1113/jphysiol.2012.248047. Epub 2013 Feb 11. J Physiol. 2013. PMID: 23401614 Free PMC article.
-
Nociceptin/orphanin FQ (N/OFQ) inhibits excitatory and inhibitory synaptic signaling in the suprachiasmatic nucleus (SCN).Neuroscience. 2005;132(4):955-65. doi: 10.1016/j.neuroscience.2004.11.057. Neuroscience. 2005. PMID: 15857701
-
Presynaptic GABA(B) receptors regulate retinohypothalamic tract synaptic transmission by inhibiting voltage-gated Ca2+ channels.J Neurophysiol. 2006 Jun;95(6):3727-41. doi: 10.1152/jn.00909.2005. J Neurophysiol. 2006. PMID: 16709723
-
Photic entrainment of circadian rhythms in rodents.Chronobiol Int. 1998 Sep;15(5):395-423. doi: 10.3109/07420529808998699. Chronobiol Int. 1998. PMID: 9787933 Review.
-
Dynamic endocannabinoid-mediated neuromodulation of retinal circadian circuitry.Ageing Res Rev. 2024 Aug;99:102401. doi: 10.1016/j.arr.2024.102401. Epub 2024 Jul 3. Ageing Res Rev. 2024. PMID: 38964508 Review.
Cited by
-
M1-Type, but Not M4-Type, Melanopsin Ganglion Cells Are Physiologically Tuned to the Central Circadian Clock.Front Neurosci. 2021 May 6;15:652996. doi: 10.3389/fnins.2021.652996. eCollection 2021. Front Neurosci. 2021. PMID: 34025341 Free PMC article.
-
Effects of Different Light Sources on Neural Activity of the Paraventricular Nucleus in the Hypothalamus.Medicina (Kaunas). 2019 Nov 9;55(11):732. doi: 10.3390/medicina55110732. Medicina (Kaunas). 2019. PMID: 31717519 Free PMC article.
-
Circadian Behavioral Responses to Light and Optic Chiasm-Evoked Glutamatergic EPSCs in the Suprachiasmatic Nucleus of ipRGC Conditional vGlut2 Knock-Out Mice.eNeuro. 2018 May 10;5(3):ENEURO.0411-17.2018. doi: 10.1523/ENEURO.0411-17.2018. eCollection 2018 May-Jun. eNeuro. 2018. PMID: 29756029 Free PMC article.
-
GABA transporters regulate tonic and synaptic GABAA receptor-mediated currents in the suprachiasmatic nucleus neurons.J Neurophysiol. 2017 Dec 1;118(6):3092-3106. doi: 10.1152/jn.00194.2017. Epub 2017 Aug 30. J Neurophysiol. 2017. PMID: 28855287 Free PMC article.
-
Diurnal Expression of the Per2 Gene and Protein in the Lateral Habenular Nucleus.Int J Mol Sci. 2015 Jul 23;16(8):16740-9. doi: 10.3390/ijms160816740. Int J Mol Sci. 2015. PMID: 26213916 Free PMC article.
References
-
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science 275: 220–224, 1997. - PubMed
-
- Alamilla J, Aguilar-Roblero R. Glutamate and GABA neurotransmission from the paraventricular thalamus to the suprachiasmatic nuclei in the rat. J Biol Rhythms 25: 28–36, 2010. - PubMed
-
- Albers HE, Lydic R, Moore-Ede MC. Entrainment and masking of circadian drinking rhythms in primates: influence of light intensity. Physiol Behav 28: 205–211, 1982. - PubMed
-
- Bailey CH, Giustetto M, Huang YY, Hawkins RD, Kandel ER. Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory? Nat Rev Neurosci 1: 11–20, 2000. - PubMed
-
- Baver SB, Pickard GE, Sollars PJ, Pickard GE. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci 27: 1763–1770, 2008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

