Dendritic HCN channels shape excitatory postsynaptic potentials at the inner hair cell afferent synapse in the mammalian cochlea
- PMID: 20220080
- PMCID: PMC2867566
- DOI: 10.1152/jn.00506.2009
Dendritic HCN channels shape excitatory postsynaptic potentials at the inner hair cell afferent synapse in the mammalian cochlea
Abstract
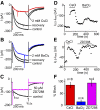
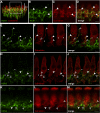
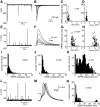
Synaptic transmission at the inner hair cell (IHC) afferent synapse, the first synapse in the auditory pathway, is specialized for rapid and reliable signaling. Here we investigated the properties of a hyperpolarization-activated current (I(h)), expressed in the afferent dendrite of auditory nerve fibers, and its role in shaping postsynaptic activity. We used whole cell patch-clamp recordings from afferent dendrites directly where they contact the IHC in excised postnatal rat cochlear turns. Excitatory postsynaptic potentials (EPSPs) of variable amplitude (1-35 mV) were found with 10-90% rise times of about 1 ms and time constants of decay of about 5 ms at room temperature. Current-voltage relations recorded in afferent dendrites revealed I(h). The pharmacological profile and reversal potential (-45 mV) indicated that I(h) is mediated by hyperpolarization-activated cyclic nucleotide-gated cation (HCN) channels. The HCN channel subunits HCN1, HCN2, and HCN4 were found to be expressed in afferent dendrites using immunolabeling. Raising intracellular cAMP levels sped up the activation kinetics, increased the magnitude of I(h) and shifted the half activation voltage (V(half)) to more positive values (-104 +/- 3 to -91 +/- 2 mV). Blocking I(h) with 50 microM ZD7288 resulted in hyperpolarization of the resting membrane potential (approximately 4 mV) and slowing the decay of the EPSP by 47%, suggesting that I(h) is active at rest and shortens EPSPs, thereby potentially improving rapid and reliable signaling at this first synapse in the auditory pathway.
Figures




References
-
- Bakondi G, Por A, Kovacs I, Szucs G, Rusznak Z. Hyperpolarization-activated, cyclic nucleotide-gated, cation non-selective channel subunit expression pattern of guinea-pig spiral ganglion cells. Neuroscience 158: 1469–1477, 2009. - PubMed
-
- Bal R, Oertel D. Hyperpolarization-activated, mixed-cation current (Ih) in octopus cells of the mammalian cochlear nucleus. J Neurophysiol 84: 806–817, 2000. - PubMed
-
- Bal R, Oertel D. Potassium currents in octopus cells of the mammalian cochlear nucleus. J Neurophysiol 86: 2299–2311, 2001. - PubMed
-
- Banks MI, Pearce RA, Smith PH. Hyperpolarization-activated cation current (Ih) in neurons of the medial nucleus of the trapezoid body: voltage-clamp analysis and enhancement by norepinephrine and cAMP suggest a modulatory mechanism in the auditory brain stem. J Neurophysiol 70: 1420–1432, 1993. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

