Impaired glial glutamate uptake induces extrasynaptic glutamate spillover in the spinal sensory synapses of neuropathic rats
- PMID: 20220084
- PMCID: PMC2867569
- DOI: 10.1152/jn.00013.2010
Impaired glial glutamate uptake induces extrasynaptic glutamate spillover in the spinal sensory synapses of neuropathic rats
Abstract
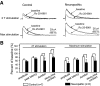
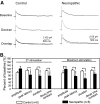
Glial cell dysfunction and excessive glutamate receptor activation in spinal dorsal horn neurons are hallmark mechanisms of pathological pain. The way in which glial cell dysfunction leads to excessive glutamate receptor activation in the spinal sensory synapses remains unknown. We and others recently reported the downregulation of glial glutamate transporter (GT) protein expression in the spinal dorsal horn of neuropathic rats. In this study, we showed that excitatory postsynaptic currents originating from N-methyl-d-aspartate receptor activation (NMDA EPSCs) elicited by peripheral synaptic input in the spinal sensory synapses were enhanced in neuropathic rats with mechanical allodynia induced by partial sciatic nerve ligation. The enhanced NMDA EPSCs were accompanied by an increased proportion of NR2B receptor activation. Physically blocking the extrasynaptic glutamate with dextran or chemically scavenging the glutamate with glutamic-pyruvic transaminase ameliorated the abnormal NMDA EPSCs in neuropathic rats. Pharmacological blockade of glial GTs with dihydrokainic acid enhanced NMDA receptor activation elicited by synaptic input or puffed glutamate in normal control rats, but this effect was precluded in neuropathic rats. Thus extrasynaptic glutamate spillover and extrasynaptic NMDA receptor activation induced by deficient glial glutamate uptake in the synapses resulted in the excessive activation of NMDA receptors in neuropathic rats. It is suggested that extrasynaptic glutamate spillover may be a key synaptic mechanism related to phenotypic alterations induced by nerve injury in the spinal dorsal horn and that glial GTs are potential new targets in the development of analgesics.
Figures




References
-
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. GLIA 32: 1–14, 2000. - PubMed
-
- Baba H, Doubell TP, Moore KA, Woolf CJ. Silent NMDA receptor-mediated synapses are developmentally regulated in the dorsal horn of the rat spinal cord. J Neurophysiol 83: 955–962, 2000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

