Plasmacytoid dendritic cells enhance mortality during lethal influenza infections by eliminating virus-specific CD8 T cells
- PMID: 20220091
- PMCID: PMC2851488
- DOI: 10.4049/jimmunol.0902984
Plasmacytoid dendritic cells enhance mortality during lethal influenza infections by eliminating virus-specific CD8 T cells
Abstract
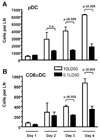
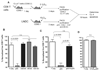
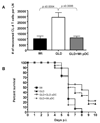
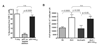
Previous studies have shown that the reduction in CD8 T cell immunity observed during high-dose influenza A virus (IAV) infection is mediated via lymph node (LN) dendritic cells (DCs) that express Fas ligand (FasL) and drive FasL-Fas (DC-T)-induced apoptosis. However, the specific DC subset(s) within the LN and the additional factors required for DC-mediated elimination of IAV-specific CD8 T cells remain unknown. In this paper, we demonstrate that plasmacytoid DCs (pDCs), which downregulate FasL during sublethal, but not lethal, IAV infection, accumulate to greater numbers within the LNs of lethal dose-infected mice. Further our findings show that pDCs from lethal, but not sublethal, dose IAV infections drive elimination of Fas(+) CD8 T cells and that this elimination occurs only in the absence of TCR recognition of IAV peptide-MHC class I complexes. Together, these results suggest that pDCs play a heretofore unknown deleterious role during lethal dose IAV infections by limiting the CD8 T cell response.
Figures


References
-
- Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J. Immunol. 1997;159:5197–5200. - PubMed
-
- GeurtsvanKessel CH, Willart MA, van Rijt LS, Muskens F, Kool M, Baas C, Thielemans K, Bennett C, Clausen BE, Hoogsteden HC, Osterhaus AD, Rimmelzwaan GF, Lambrecht BN. Clearance of influenza virus from the lung depends on migratory langerin+CD11b− but not plasmacytoid dendritic cells. J. Exp. Med. 2008;205:1621–1634. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

