Absence of Epstein-Barr virus in the brain and CSF of patients with multiple sclerosis
- PMID: 20220124
- PMCID: PMC2865779
- DOI: 10.1212/WNL.0b013e3181d865a1
Absence of Epstein-Barr virus in the brain and CSF of patients with multiple sclerosis
Abstract
Objective: Epstein-Barr virus (EBV) is a ubiquitous human herpesvirus that becomes latent in B-lymphocytes and has been implicated in the pathogenesis of multiple sclerosis (MS). We searched for latent and active EBV infection in MS brain and CSF.
Methods: Nested and non-nested real-time PCR were used to detect cell-specific and EBV-specific transcripts in 15 fresh-frozen and 5 formalin-fixed paraffin-embedded MS plaques and in single MS CSF B-lymphocytes and plasma cells. Intrathecal anti-EBV antibody synthesis was measured by ELISA. Immunocytochemistry was used to detect binding of MS CSF and recombinant antibodies (rAbs) generated from clonally expanded plasma cells in MS CSF to EBV-infected cells.
Results: No EBV RNA was found in MS CSF B-lymphocytes or plasma cells. In active MS plaques, EBV-encoded RNA (EBER)-1 was the only and rarely detected transcript. The frequency of detected intrathecal anti-EBV antibody synthesis in patients with MS did not differ from that in non-MS inflammatory CNS disease control patients. Anti-EBV antibodies were detected in the CSF of patients with MS, but MS rAbs did not react with EBV.
Conclusions: Application of real-time PCR to multiple sclerosis brain and single B-lymphocytes in CSF did not reveal any evidence of active Epstein-Barr virus infection.
Figures

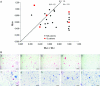
Comment in
-
EBV and brain matter(s)?Neurology. 2010 Apr 6;74(14):1092-5. doi: 10.1212/WNL.0b013e3181dabfb5. Epub 2010 Mar 17. Neurology. 2010. PMID: 20237306 No abstract available.
-
Epstein-Barr virus, 9.4 T MRI and phosphodiesterase inhibitors in multiple sclerosis.J Neurol. 2010 May;257(5):860-2. doi: 10.1007/s00415-010-5571-y. J Neurol. 2010. PMID: 20419309 No abstract available.
References
-
- Henle W, Henle G. Epstein-Barr virus and infectious mononucleosis. N Engl J Med 1973;288:263–264. - PubMed
-
- de-Thé G. Epstein-Barr virus behavior in different populations and implications for control of Epstein-Barr virus–associated tumors. Cancer Res 1976;36:692–695. - PubMed
-
- Ascherio A, Munger K. Environmental risk factors for multiple sclerosis: part I: the role of infection. Ann Neurol 2007;61:288–299. - PubMed
-
- Ascherio A, Munch M. Epstein-Barr virus and multiple sclerosis. Epidemiology 2000;11:220–224. - PubMed
-
- Ascherio A, Munger K, Lennette E, et al. Epstein-Barr virus antibodies and risk of multiple sclerosis: a prospective study. JAMA 2001;286:3083–3088. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
