The cancer/testis antigen CAGE with oncogenic potential stimulates cell proliferation by up-regulating cyclins D1 and E in an AP-1- and E2F-dependent manner
- PMID: 20220142
- PMCID: PMC2863237
- DOI: 10.1074/jbc.M109.084400
The cancer/testis antigen CAGE with oncogenic potential stimulates cell proliferation by up-regulating cyclins D1 and E in an AP-1- and E2F-dependent manner
Abstract
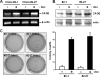
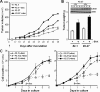
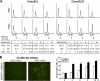
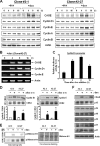
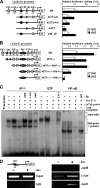
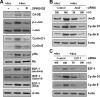
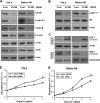
A cancer/testis antigen, CAGE, is widely expressed in various cancer tissues and cancer cell lines but not in normal tissues except the testis. In the present study, ectopic expression of CAGE in fibroblast cells resulted in foci formation, suggesting its cell-transforming ability. Using stable HeLa transfectant clones with the tetracycline-inducible CAGE gene, we found that CAGE overexpression stimulated both anchorage-dependent and -independent cell growth in vitro and promoted tumor growth in a xenograft mouse model. Cell cycle analysis showed that CAGE augments the levels of cyclin D1 and E, thereby activating cyclin-associated cyclin-dependent kinases and subsequently accelerating the G(1) to S progression. Moreover, increased cyclin D1 and E levels in CAGE-overexpressing cells were observed even in a growth arrested state, indicating a direct effect of CAGE on G(1) cyclin expression. CAGE-induced expression of cyclins D1 and E was found to be mediated by AP-1 and E2F-1 transcription factors, and among the AP-1 members, c-Jun and JunD appeared to participate in CAGE-mediated up-regulation of cyclin D1. CAGE overexpression also enhanced retinoblastoma phosphorylation and subsequent E2F-1 nuclear translocation. In contrast, small interfering RNA-mediated knockdown of CAGE suppressed the expression of G(1) cyclins, activation of AP-1 and E2F-1, and cell proliferation in both HeLa cervical cancer cells and Malme-3M melanoma cells. These results suggest that the cancer/testis antigen CAGE possesses oncogenic potential and promotes cell cycle progression by inducing AP-1- and E2F-dependent expression of cyclins D1 and E.
Figures

References
-
- Boon T., Old L. J. (1997) Curr. Opin. Immunol. 9, 681–683 - PubMed
-
- Scanlan M. J., Gure A. O., Jungbluth A. A., Old L. J., Chen Y. T. (2002) Immunol. Rev. 188, 22–32 - PubMed
-
- Scanlan M. J., Simpson A. J., Old L. J. (2004) Cancer Immun. 4, 1. - PubMed
-
- Zendman A. J., Ruiter D. J., Van Muijen G. N. (2003) J. Cell. Physiol. 194, 272–288 - PubMed
-
- van der Bruggen P., Traversari C., Chomez P., Lurquin C., De Plaen E., Van den Eynde B., Knuth A., Boon T. (1991) Science 254, 1643–1647 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

