Hemorrhage-induced intestinal damage is complement-independent in Helicobacter hepaticus-infected mice
- PMID: 20220569
- PMCID: PMC2998760
- DOI: 10.1097/SHK.0b013e3181dc077e
Hemorrhage-induced intestinal damage is complement-independent in Helicobacter hepaticus-infected mice
Abstract
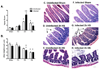
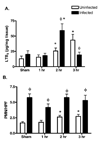
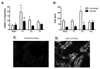
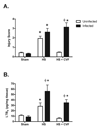
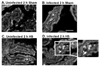
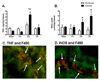
With more than half of the world population infected, Helicobacter infection is an important public health issue associated with gastrointestinal cancers and inflammatory bowel disease. Animal studies indicate that complement and oxidative stress play a role in Helicobacter infections. Hemorrhage (HS) induces tissue damage that is attenuated by blockade of either complement activation or oxidative stress products. Therefore, we hypothesized that chronic Helicobacter hepaticus infection would modulate HS-induced intestinal damage and inflammation. To test this hypothesis, we examined HS-induced jejunal damage and inflammation in uninfected and H. hepaticus-infected mice. Helicobacter hepaticus infection increased HS-induced midjejunal mucosal damage despite attenuating complement activation. In addition, infection alone increased chemokine secretion, changing the HS-induced neutrophil infiltration to a macrophage-mediated inflammatory response. The HS-induced macrophage infiltration correlated with increased secretion of tumor necrosis factor-α and nitric oxide in the infected mice. Together, these data indicate that Helicobacter infection modulates the mechanism of HS-induced intestinal damage and inflammation from a complement-mediated response to a macrophage response with elevated tumor necrosis factor-α and nitric oxide. These data indicate that chronic low-level infections change the response to trauma and should be considered when designing and administering therapeutics.
Figures

Similar articles
-
Helicobacter hepaticus-induced colitis in interleukin-10-deficient mice: cytokine requirements for the induction and maintenance of intestinal inflammation.Infect Immun. 2001 Jul;69(7):4232-41. doi: 10.1128/IAI.69.7.4232-4241.2001. Infect Immun. 2001. PMID: 11401959 Free PMC article.
-
Helicobacter hepaticus infection triggers inflammatory bowel disease in T cell receptor alphabeta mutant mice.Comp Med. 2000 Dec;50(6):586-94. Comp Med. 2000. PMID: 11200563
-
Sex influence on chronic intestinal inflammation in Helicobacter hepaticus-infected A/JCr mice.Comp Med. 2004 Jun;54(3):301-8. Comp Med. 2004. PMID: 15253277
-
Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer.Mucosal Immunol. 2011 Jan;4(1):22-30. doi: 10.1038/mi.2010.61. Epub 2010 Oct 13. Mucosal Immunol. 2011. PMID: 20944559 Free PMC article. Review.
-
Liver tumorigenesis by Helicobacter hepaticus: considerations of mechanism.In Vivo. 1996 May-Jun;10(3):285-92. In Vivo. 1996. PMID: 8797029 Review.
Cited by
-
Macrophage-produced IL-12p70 mediates hemorrhage-induced damage in a complement-dependent manner.Shock. 2011 Feb;35(2):134-40. doi: 10.1097/SHK.0b013e3181ed8ec9. Shock. 2011. PMID: 20577145 Free PMC article.
-
Innate immunity and immunotherapy for hemorrhagic shock.Front Immunol. 2022 Aug 25;13:918380. doi: 10.3389/fimmu.2022.918380. eCollection 2022. Front Immunol. 2022. PMID: 36091025 Free PMC article. Review.
-
Helicobacter infection alters MyD88 and Trif signalling in response to intestinal ischaemia-reperfusion.Exp Physiol. 2011 Feb;96(2):104-13. doi: 10.1113/expphysiol.2010.055426. Epub 2010 Nov 5. Exp Physiol. 2011. PMID: 21056969 Free PMC article.
References
-
- Malaty HM. Epidemiology of Helicobacter pylori infection. Best Pract Res Clin Gastroenterol. 2007;21:205–214. - PubMed
-
- Veijola L, Nilsson I, Halme L, Al-Soud WA, Mäkinen J, Ljungh A, Rautelin H. Detection of Helicobacter species in chronic liver disease and chronic inflammatory bowel disease. Ann Med. 2007;39:554–560. - PubMed
-
- Myles MH, Livingston RS, Franklin CL. Pathogenicity of Helicobacter rodentium in A/JCr and SCID mice. Comp Med. 2004;54:549–557. - PubMed
-
- Chaouche-Drider N, Kaparakis M, Karrar A, Fernandez MI, Carneiro LA, Viala J, Boneca IG, Moran AP, Philpott DJ, Ferrero RL. A commensal Helicobacter sp. of the rodent intestinal flora activates TLR2 and NOD1 responses in epithelial cells. PLoS ONE. 2009;4:e5396. doi:5310.1371/journal.pone.0005396. - PMC - PubMed
-
- Myles MH, Dieckgraefe BK, Criley JM, Franklin CL. Characterization of cecal gene expression in a differentially susceptible mouse model of bacterial-induced inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:822–836. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

