Limited trafficking of a neurotropic virus through inefficient retrograde axonal transport and the type I interferon response
- PMID: 20221252
- PMCID: PMC2832671
- DOI: 10.1371/journal.ppat.1000791
Limited trafficking of a neurotropic virus through inefficient retrograde axonal transport and the type I interferon response
Abstract
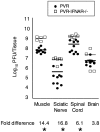
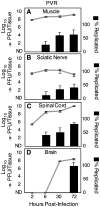
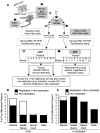
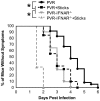
Poliovirus is an enteric virus that rarely invades the human central nervous system (CNS). To identify barriers limiting poliovirus spread from the periphery to CNS, we monitored trafficking of 10 marked viruses. After oral inoculation of susceptible mice, poliovirus was present in peripheral neurons, including vagus and sciatic nerves. To model viral trafficking in peripheral neurons, we intramuscularly injected mice with poliovirus, which follows a muscle-sciatic nerve-spinal cord-brain route. Only 20% of the poliovirus population successfully moved from muscle to brain, and three barriers limiting viral trafficking were identified. First, using light-sensitive viruses, we found limited viral replication in peripheral neurons. Second, retrograde axonal transport of poliovirus in peripheral neurons was inefficient; however, the efficiency was increased upon muscle damage, which also increased the transport efficiency of a non-viral neural tracer, wheat germ agglutinin. Third, using susceptible interferon (IFN) alpha/beta receptor knockout mice, we demonstrated that the IFN response limited viral movement from the periphery to the brain. Surprisingly, the retrograde axonal transport barrier was equivalent in strength to the IFN barrier. Illustrating the importance of barriers created by the IFN response and inefficient axonal transport, IFN alpha/beta receptor knockout mice with muscle damage permitted 80% of the viral population to access the brain, and succumbed to disease three times faster than mice with intact barriers. These results suggest that multiple separate barriers limit poliovirus trafficking from peripheral neurons to the CNS, possibly explaining the rare incidence of paralytic poliomyelitis. This study identifies inefficient axonal transport as a substantial barrier to poliovirus trafficking in peripheral neurons, which may limit CNS access for other viruses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

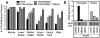
Similar articles
-
Comparison of three neurotropic viruses reveals differences in viral dissemination to the central nervous system.Virology. 2016 Jan;487:1-10. doi: 10.1016/j.virol.2015.09.019. Epub 2015 Oct 16. Virology. 2016. PMID: 26479325 Free PMC article.
-
Retrograde transport of intact poliovirus through the axon via the fast transport system.Virology. 1998 Oct 10;250(1):67-75. doi: 10.1006/viro.1998.9360. Virology. 1998. PMID: 9770421
-
Gene expression in the muscle and central nervous system following intramuscular inoculation of encapsidated or naked poliovirus replicons.Virology. 2003 Sep 15;314(1):45-61. doi: 10.1016/s0042-6822(03)00385-4. Virology. 2003. PMID: 14517059
-
[Analysis of dissemination pathways for poliovirus].Uirusu. 2009 Jun;59(1):107-14. doi: 10.2222/jsv.59.107. Uirusu. 2009. PMID: 19927995 Review. Japanese.
-
Innate host barriers to viral trafficking and population diversity: lessons learned from poliovirus.Adv Virus Res. 2010;77:85-118. doi: 10.1016/B978-0-12-385034-8.00004-1. Adv Virus Res. 2010. PMID: 20951871 Free PMC article. Review.
Cited by
-
Mechanisms controlling virulence thresholds of mixed viral populations.J Virol. 2011 Oct;85(19):9778-88. doi: 10.1128/JVI.00355-11. Epub 2011 Jul 27. J Virol. 2011. PMID: 21795346 Free PMC article.
-
The reovirus sigma1s protein is a determinant of hematogenous but not neural virus dissemination in mice.J Virol. 2011 Nov;85(22):11781-90. doi: 10.1128/JVI.02289-10. Epub 2011 Sep 14. J Virol. 2011. PMID: 21917967 Free PMC article.
-
Axonal spread of neuroinvasive viral infections.Trends Microbiol. 2015 May;23(5):283-8. doi: 10.1016/j.tim.2015.01.002. Epub 2015 Jan 29. Trends Microbiol. 2015. PMID: 25639651 Free PMC article. Review.
-
Trans-endocytosis elicited by nectins transfers cytoplasmic cargo, including infectious material, between cells.J Cell Sci. 2019 Aug 23;132(16):jcs235507. doi: 10.1242/jcs.235507. J Cell Sci. 2019. PMID: 31331966 Free PMC article.
-
Molecular Pathogenicity of Enteroviruses Causing Neurological Disease.Front Microbiol. 2020 Apr 9;11:540. doi: 10.3389/fmicb.2020.00540. eCollection 2020. Front Microbiol. 2020. PMID: 32328043 Free PMC article. Review.
References
-
- Miranda-Saksena M, Boadle RA, Aggarwal A, Tijono B, Rixon FJ, et al. Herpes simplex virus utilizes the large secretory vesicle pathway for anterograde transport of tegument and envelope proteins and for viral exocytosis from growth cones of human fetal axons. J Virol. 2009;83:3187–3199. - PMC - PubMed
-
- Mueller S, Cao X, Welker R, Wimmer E. Interaction of the poliovirus receptor CD155 with the dynein light chain Tctex-1 and its implication for poliovirus pathogenesis. J Biol Chem. 2002;277:7897–7904. - PubMed
-
- Bodian D. Viremia in experimental poliomyelitis. II. Viremia and the mechanism of the provoking effect of injections or trauma. Am J Hyg. 1954;60:358–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

