Mechanical Stretch-Induced Protection against Myocardial Ischemia-Reperfusion Injury Involves AMP-Activated Protein Kinase
- PMID: 20221273
- PMCID: PMC2835976
- DOI: 10.4196/kjpp.2010.14.1.1
Mechanical Stretch-Induced Protection against Myocardial Ischemia-Reperfusion Injury Involves AMP-Activated Protein Kinase
Abstract
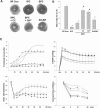
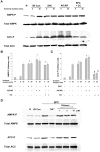
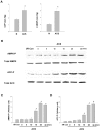
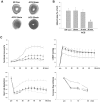
AMP-activated protein kinase (AMPK) protects various tissues and cells from ischemic insults and is activated by many stimuli including mechanical stretch. Therefore, this study investigated if the activation of AMPK is involved in stretch-induced cardioprotection (SIC). Intraventricular balloon and aorto-caval shunt (ACS) were used to stretch rat hearts ex vivo and in vivo, respectively. Stretch preconditioning reduced myocardial infarct induced by ischemia-reperfusion (I/R) and improved post-ischemic functional recovery. Phosphorylation of AMPK and its downstream substrate, acetyl-CoA carboxylase (ACC) were increased by mechanical stretch and ACC phosphorylation was completely blocked by the AMPK inhibitor, Compound C. AMPK activator (AICAR) mimicked SIC. Gadolinium, a blocker of stretch-activated ion channels (SACs), inhibited the stretch-induced phosphorylation of AMPK and ACC, whereas diltiazem, a specific L-type calcium channel blocker, did not affect AMPK activation. Furthermore, SIC was abrogated by Compound C and gadolinium. The in vivo stretch induced by ACS increased AMPK activation and reduced myocardial infarct. These findings indicate that stretch preconditioning can induce the cardioprotection against I/R injury, and activation of AMPK plays an important role in SIC, which might be mediated by SACs.
Keywords: AMP-activated protein kinase; Cardioprotection; Ischemia-reperfusion; Stretch.
Figures

References
-
- Das DK, Maulik N. Cardiac genomic response following preconditioning stimulus. Cardiovasc Res. 2006;70:254–263. - PubMed
-
- Shintani-Ishida K, Nakajima M, Uemura K, Yoshida K. Ischemic preconditioning protects cardiomyocytes against ischemic injury by inducing GRP78. Biochem Biophys Res Commun. 2006;345:1600–1605. - PubMed
-
- Kim YH, Kim CH, Kim GT, Kim IK, Park JW, Kim MS. Involvement of adenosine in cardioprotective effect of catecholamine preconditioning in ischemia-reperfused heart of rat. Korean J Physiol Pharmacol. 1998;2:753–761.
-
- Jiao JD, Garg V, Yang B, Hu K. Novel functional role of heat shock protein 90 in ATP-sensitive K+ channel-mediated hypoxic preconditioning. Cardiovasc Res. 2008;77:126–133. - PubMed
-
- Ovize M, Kloner RA, Przyklenk K. Stretch preconditions canine myocardium. Am J Physiol. 1994;266:H137–H146. - PubMed

