Calcium sensitization induced by sodium fluoride in permeabilized rat mesenteric arteries
- PMID: 20221280
- PMCID: PMC2835983
- DOI: 10.4196/kjpp.2010.14.1.51
Calcium sensitization induced by sodium fluoride in permeabilized rat mesenteric arteries
Abstract
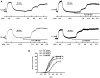
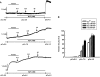
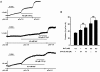
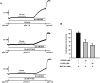
It was hypothesized that NaF induces calcium sensitization in Ca(2+)-controlled solution in permeabilized rat mesenteric arteries. Rat mesenteric arteries were permeabilized with beta-escin and subjected to tension measurement. NaF potentiated the concentration-response curves to Ca(2+) (decreased EC(50) and increased E(max)). Cumulative addition of NaF (4.0, 8.0 and 16 mM) also increased vascular tension in Ca(2+)-controlled solution at pCa 7.0 or pCa 6.5, but not at pCa 8.0. NaF-induced vasocontraction and GTPgammaS-induced vasocontraction were not additive. NaF-induced vasocontraction at pCa 7.0 was inhibited by pretreatment with Rho kinase inhibitors H1152 or Y27632 but not with a MLCK inhibitor ML-7 or a PKC inhibitor Ro31-8220. NaF induces calcium sensitization in a Ca(2+)-dependent manner in beta-escin-permeabilized rat mesenteric arteries. These results suggest that NaF is an activator of the Rho kinase signaling pathway during vascular contraction.
Keywords: Calcium sensitization; Mesenteric artery; Permeabilization; Rho kinase; Sodium fluoride.
Figures

Similar articles
-
3',4'-Dihydroxyflavonol reduces vascular contraction through Ca²⁺ desensitization in permeabilized rat mesenteric artery.Naunyn Schmiedebergs Arch Pharmacol. 2012 Feb;385(2):191-202. doi: 10.1007/s00210-011-0697-8. Epub 2011 Oct 14. Naunyn Schmiedebergs Arch Pharmacol. 2012. PMID: 21993847
-
Fluoride induces vascular contraction through activation of RhoA/Rho kinase pathway in isolated rat aortas.Environ Toxicol Pharmacol. 2010 May;29(3):290-6. doi: 10.1016/j.etap.2010.02.004. Epub 2010 Mar 11. Environ Toxicol Pharmacol. 2010. PMID: 21787615
-
A role for Rho kinase in vascular contraction evoked by sodium fluoride.Biochem Biophys Res Commun. 2006 Apr 28;343(1):27-33. doi: 10.1016/j.bbrc.2006.02.120. Epub 2006 Feb 28. Biochem Biophys Res Commun. 2006. PMID: 16527249
-
Calcium sensitivity and cooperativity of permeabilized rat mesenteric lymphatics.Am J Physiol Regul Integr Comp Physiol. 2008 May;294(5):R1524-32. doi: 10.1152/ajpregu.00888.2007. Epub 2008 Feb 27. Am J Physiol Regul Integr Comp Physiol. 2008. PMID: 18305021
-
Enhanced contractile responses of arteries from streptozotocin diabetic rats to sodium fluoride.Br J Pharmacol. 1996 May;118(1):115-22. doi: 10.1111/j.1476-5381.1996.tb15373.x. Br J Pharmacol. 1996. PMID: 8733583 Free PMC article.
Cited by
-
Advances in the Utilization of Tea Polysaccharides: Preparation, Physicochemical Properties, and Health Benefits.Polymers (Basel). 2022 Jul 6;14(14):2775. doi: 10.3390/polym14142775. Polymers (Basel). 2022. PMID: 35890551 Free PMC article. Review.
-
3',4'-Dihydroxyflavonol reduces vascular contraction through Ca²⁺ desensitization in permeabilized rat mesenteric artery.Naunyn Schmiedebergs Arch Pharmacol. 2012 Feb;385(2):191-202. doi: 10.1007/s00210-011-0697-8. Epub 2011 Oct 14. Naunyn Schmiedebergs Arch Pharmacol. 2012. PMID: 21993847
References
-
- Hirano K. Current topics in the regulatory mechanism underlying the Ca2+ sensitization of the contractile apparatus in vascular smooth muscle. J Pharmacol Sci. 2007;104:109–115. - PubMed
-
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. - PubMed
-
- Budzyn K, Marley PD, Sobey CG. Targeting Rho and Rho-kinase in the treatment of cardiovascular disease. Trends Pharmacol Sci. 2006;27:97–104. - PubMed
-
- Murthy KS, Makhlouf GM. Fluoride activates G protein-dependent and -independent pathways in dispersed intestinal smooth muscle cells. Biochem Biophys Res Commun. 1994;202:1681–1687. - PubMed
-
- Bogatcheva NV, Wang P, Birukova AA, Verin AD, Garcia JG. Mechanism of fluoride-induced MAP kinase activation in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1139–L1145. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

