Microinjection of IL-1β into the trigeminal transition zone produces bilateral NMDA receptor-dependent orofacial hyperalgesia involving descending circuitry
- PMID: 20221418
- PMCID: PMC2835306
- DOI: 10.2174/1876386300902010076
Microinjection of IL-1β into the trigeminal transition zone produces bilateral NMDA receptor-dependent orofacial hyperalgesia involving descending circuitry
Abstract
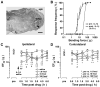
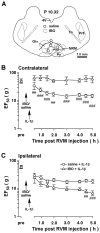
Our recent studies indicate that the prototypic proinflammatory cytokine IL-1β is upregulated in astroglial cells in the trigeminal interplolaris/caudalis (Vi/Vc) transition zone, a region of the spinal trigeminal complex involved in trigeminal pain processing, after masseter muscle inflammation. Here we investigated the effect of microinjection of IL-1β into the Vi/Vc transition zone on orofacial nociception. The mechanical sensitivity of the orofacial site was assessed with von Frey microfilaments. The EF(50) values, defined as the von Frey filament force (g) that produces a 50% response frequency, were derived and used as a measure of mechanical sensitivity. A significant reduction in EF(50) indicates the occurrence of mechanical hyperalgesia/allodynia. Unilateral intra-Vi/Vc IL-1β (0.016-160 fmol) produced hyperalgesia/allodynia dose-dependently, which appeared at bilateral facial sites. The hyperalgesia was detectable as early as 30 min and lasted for 2-6 h (n=6, p<0.01). Intra-Vi/Vc pretreatment with an IL-1receptor antagonist (1 nmol) attenuated the IL-1β-induced hyperalgesia (p<0.01). Pre-injection of AP-5 (10 pmol) and MK-801 (20 pmol), two NMDA receptor antagonists, significantly attenuated IL-1β-induced hyperalgesia (p<0.05). Pretreatment with glial inhibitors fluorocitrate (120 pmol), minocycline (200 pmol) and propentofylline (10 pmol) did not attenuate IL-1β-induced hyperalgesia. Excitotoxic lesions of the rostral ventromedial medulla with ibotenic acid (2 μg) abolished IL-1β-induced contralateral hyperalgesia, suggesting a contribution of descending facilitatory drive. These results suggest that the IL-1β-produced effect on nociception was downstream to glial activation and involves interaction with NMDA receptors.
Figures


Similar articles
-
Trigeminal-rostral ventromedial medulla circuitry is involved in orofacial hyperalgesia contralateral to tissue injury.Mol Pain. 2012 Oct 23;8:78. doi: 10.1186/1744-8069-8-78. Mol Pain. 2012. PMID: 23092240 Free PMC article.
-
Differential involvement of trigeminal transition zone and laminated subnucleus caudalis in orofacial deep and cutaneous hyperalgesia: the effects of interleukin-10 and glial inhibitors.Mol Pain. 2009 Dec 21;5:75. doi: 10.1186/1744-8069-5-75. Mol Pain. 2009. PMID: 20025765 Free PMC article.
-
Trigeminal transition zone/rostral ventromedial medulla connections and facilitation of orofacial hyperalgesia after masseter inflammation in rats.J Comp Neurol. 2005 Dec 26;493(4):510-23. doi: 10.1002/cne.20797. J Comp Neurol. 2005. PMID: 16304628
-
IL-1beta in the trigeminal subnucleus caudalis contributes to extra-territorial allodynia/hyperalgesia following a trigeminal nerve injury.Eur J Pain. 2011 May;15(5):467.e1-14. doi: 10.1016/j.ejpain.2010.10.006. Epub 2010 Nov 18. Eur J Pain. 2011. PMID: 21093329
-
The role of trigeminal interpolaris-caudalis transition zone in persistent orofacial pain.Int Rev Neurobiol. 2011;97:207-25. doi: 10.1016/B978-0-12-385198-7.00008-4. Int Rev Neurobiol. 2011. PMID: 21708312 Free PMC article. Review.
Cited by
-
Trigeminal nerve injury-induced thrombospondin-4 up-regulation contributes to orofacial neuropathic pain states in a rat model.Eur J Pain. 2014 Apr;18(4):489-95. doi: 10.1002/j.1532-2149.2013.00396.x. Epub 2013 Sep 9. Eur J Pain. 2014. PMID: 24019258 Free PMC article.
-
Nociceptive behavioural assessments in mouse models of temporomandibular joint disorders.Int J Oral Sci. 2020 Sep 29;12(1):26. doi: 10.1038/s41368-020-00095-0. Int J Oral Sci. 2020. PMID: 32989215 Free PMC article.
-
NMDARs mediate peripheral and central sensitization contributing to chronic orofacial pain.Front Cell Neurosci. 2022 Sep 27;16:999509. doi: 10.3389/fncel.2022.999509. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36238833 Free PMC article. Review.
-
The role of P2X3 receptors in bilateral masseter muscle allodynia in rats.Croat Med J. 2016 Dec 31;57(6):530-539. doi: 10.3325/cmj.2016.57.530. Croat Med J. 2016. PMID: 28051277 Free PMC article.
-
Neuron-glia crosstalk gets serious: role in pain hypersensitivity.Curr Opin Anaesthesiol. 2008 Oct;21(5):570-9. doi: 10.1097/ACO.0b013e32830edbdf. Curr Opin Anaesthesiol. 2008. PMID: 18784481 Free PMC article. Review.
References
-
- Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med. 2005;257:139–55. - PubMed
-
- Dinarello CA. Therapeutic strategies to reduce IL-1 activity in treating local and systemic inflammation. Curr Opin Pharmacol. 2004;4:378–85. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
