TGFbeta1 stimulates the over-production of white matter astrocytes from precursors of the "brain marrow" in a rodent model of neonatal encephalopathy
- PMID: 20221422
- PMCID: PMC2832687
- DOI: 10.1371/journal.pone.0009567
TGFbeta1 stimulates the over-production of white matter astrocytes from precursors of the "brain marrow" in a rodent model of neonatal encephalopathy
Abstract
Background: In children born prematurely and those surviving cerebral ischemia there are white matter abnormalities that correlate with neurological dysfunction. Since this injury occurs in the immature brain, when the majority of subventricular zone (SVZ) cells generate white matter oligodendrocytes, we sought to study the effect this injury has on gliogenesis from the SVZ. We hypothesized that there is aberrant glial cell generation from the SVZ after neonatal hypoxia ischemia (H/I) that contributes to an increased astrogliogenesis with concomitant oligodendroglial insufficiency. Mechanistically we hypothesized that an increase in specific locally produced cytokines during recovery from injury were modifying the differentiation of glial progenitors towards astrocytes at the expense of the more developmentally-appropriate oligodendrocytes.
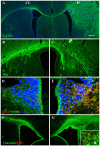
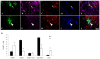
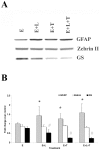
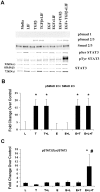
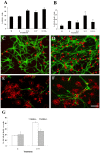
Methodology/principal finding: For these studies we used the Vannucci H/I rat model where P6 rats are subjected to unilateral common carotid ligation followed by 75 min of systemic hypoxia. Retroviral lineage tracing studies combined with morphological and immunohistochemical analyses revealed the preferential generation of SVZ-derived white matter astrocytes instead of oligodendrocytes post hypoxia/ischemia. Microarray and QRT-PCR analyses of the damaged SVZ showed increased expression of several cytokines and receptors that are known to promote astrocyte differentiation, such as EGF, LIF and TGFbeta1 signaling components. Using gliospheres to model the neonatal SVZ, we evaluated the effects of these cytokines on signal transduction pathways regulating astrocyte generation, proliferation and differentiation. These studies demonstrated that combinations of EGF, LIF and TGFbeta1 reconstituted the increased astrogliogenesis. TGFbeta1-induced Smad 2/3 phosphorylation and the combination of EGF, LIF and TGFbeta1 synergistically increased STAT3 phosphorylation over single or double cytokine combinations. Pharmacologically inhibiting ALK5 signaling in vitro antagonized the TGFbeta1-induced increase in astrocyte generation and antagonizing ALK5 signaling in vivo similarly inhibited astrogliogenesis within the SVZ during recovery from H/I.
Conclusion/significance: Altogether, these data indicate that there is aberrant specification of glial precursors within the neonatal SVZ during recovery from neonatal H/I that is a consequence of altered cytokine signaling. Our studies further suggest that antagonizing the ALK5 receptor will restore the normal pattern of cell differentiation after injury to the immature brain.
Conflict of interest statement
Figures
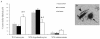

References
-
- Vannucci RC, Vannucci SJ. Perinatal hypoxic-ischemic brain damage: evolution of an animal model. Developmental Neuroscience. 2005;27:81–86. - PubMed
-
- Rice JE, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Annals of Neurology. 1981:131–141. - PubMed
-
- Welsh FA, Vannucci RC, Brierley JB. Columnar alterations of NADH fluorescence during hypoxia-ischemia in immature rat brain. Journal of Cerebral Blood Flow & Metabolism. 1982;2:221–228. - PubMed
-
- Vannucci RC, Lyons DT, Vasta F. Regional cerebral blood flow during hypoxia-ischemia in immature rats. Stroke. 1988;19:245–250. - PubMed
-
- Levison SW, Rothstein RP, Romanko MJ, Snyder MJ, Meyers RL, et al. Hypoxia/ischemia depletes the rat perinatal subventricular zone of oligodendrocyte progenitors and neural stem cells. Dev Neurosci. 2001;23:234–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

