Direct visualization by cryo-EM of the mycobacterial capsular layer: a labile structure containing ESX-1-secreted proteins
- PMID: 20221442
- PMCID: PMC2832766
- DOI: 10.1371/journal.ppat.1000794
Direct visualization by cryo-EM of the mycobacterial capsular layer: a labile structure containing ESX-1-secreted proteins
Abstract
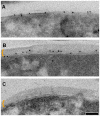
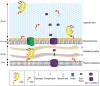
The cell envelope of mycobacteria, a group of Gram positive bacteria, is composed of a plasma membrane and a Gram-negative-like outer membrane containing mycolic acids. In addition, the surface of the mycobacteria is coated with an ill-characterized layer of extractable, non-covalently linked glycans, lipids and proteins, collectively known as the capsule, whose occurrence is a matter of debate. By using plunge freezing cryo-electron microscopy technique, we were able to show that pathogenic mycobacteria produce a thick capsule, only present when the cells were grown under unperturbed conditions and easily removed by mild detergents. This detergent-labile capsule layer contains arabinomannan, alpha-glucan and oligomannosyl-capped glycolipids. Further immunogenic and proteomic analyses revealed that Mycobacterium marinum capsule contains high amounts of proteins that are secreted via the ESX-1 pathway. Finally, cell infection experiments demonstrated the importance of the capsule for binding to cells and dampening of pro-inflammatory cytokine response. Together, these results show a direct visualization of the mycobacterial capsular layer as a labile structure that contains ESX-1-secreted proteins.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



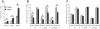
References
-
- Gagliardi MC, Lemassu A, Teloni R, Mariotti S, Sargentini V, et al. Cell wall-associated alpha-glucan is instrumental for Mycobacterium tuberculosis to block CD1 molecule expression and disable the function of dendritic cell derived from infected monocyte. Cell Microbiol. 2007;9:2081–2092. - PubMed
-
- Daffe M, Etienne G. The capsule of Mycobacterium tuberculosis and its implications for pathogenicity. Tubercle Lung Dis. 1999;79:153–169. - PubMed
-
- Minnikin DE. Lipids: complex lipids, their chemistry, biosynthesis and roles.The Biology of Mycobacteria. In: Ratledge C, Standford J, editors. The Biology of Mycobacteria. London: Academic; 1982. pp. 95–184.
-
- Brennan PJ, Crick DC. The cell-wall core of Mycobacterium tuberculosis in the context of drug discovery. Curr Top Med Chem. 2007;7:475–488. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

