Direct restriction of virus release and incorporation of the interferon-induced protein BST-2 into HIV-1 particles
- PMID: 20221443
- PMCID: PMC2832767
- DOI: 10.1371/journal.ppat.1000701
Direct restriction of virus release and incorporation of the interferon-induced protein BST-2 into HIV-1 particles
Abstract
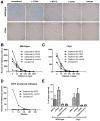
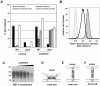
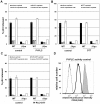
Investigation of the Vpu protein of HIV-1 recently uncovered a novel aspect of the mammalian innate response to enveloped viruses: retention of progeny virions on the surface of infected cells by the interferon-induced, transmembrane and GPI-anchored protein BST-2 (CD317; tetherin). BST-2 inhibits diverse families of enveloped viruses, but how it restricts viral release is unclear. Here, immuno-electron microscopic data indicate that BST-2 is positioned to directly retain nascent HIV virions on the plasma membrane of infected cells and is incorporated into virions. Virion-incorporation was confirmed by capture of infectivity using antibody to the ectodomain of BST-2. Consistent with a direct tethering mechanism, we confirmed that proteolysis releases restricted virions and further show that this removed the ectodomain of BST-2 from the cell surface. Unexpectedly, enzymatic cleavage of GPI anchors did not release restricted virions, weighing against models in which individual BST-2 molecules span the virion and host cell membranes. Although the exact molecular topology of restriction remains unsolved, we suggest that the incorporation of BST-2 into viral envelopes underlies its broad restrictive activity, whereas its relative exclusion from virions and sites of viral assembly by proteins such as HIV-1 Vpu may provide viral antagonism of restriction.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


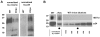
Similar articles
-
Antiviral activity of the interferon-induced cellular protein BST-2/tetherin.AIDS Res Hum Retroviruses. 2009 Dec;25(12):1197-210. doi: 10.1089/aid.2009.0253. AIDS Res Hum Retroviruses. 2009. PMID: 19929170 Free PMC article. Review.
-
The C-Terminal End of HIV-1 Vpu Has a Clade-Specific Determinant That Antagonizes BST-2 and Facilitates Virion Release.J Virol. 2019 May 15;93(11):e02315-18. doi: 10.1128/JVI.02315-18. Print 2019 Jun 1. J Virol. 2019. PMID: 30867310 Free PMC article.
-
The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein.Cell Host Microbe. 2008 Apr 17;3(4):245-52. doi: 10.1016/j.chom.2008.03.001. Epub 2008 Mar 13. Cell Host Microbe. 2008. PMID: 18342597 Free PMC article.
-
HIV-1 Vpu Antagonizes CD317/Tetherin by Adaptor Protein-1-Mediated Exclusion from Virus Assembly Sites.J Virol. 2016 Jul 11;90(15):6709-6723. doi: 10.1128/JVI.00504-16. Print 2016 Aug 1. J Virol. 2016. PMID: 27170757 Free PMC article.
-
BST-2/tetherin: a new component of the innate immune response to enveloped viruses.Trends Microbiol. 2010 Sep;18(9):388-96. doi: 10.1016/j.tim.2010.06.010. Epub 2010 Aug 3. Trends Microbiol. 2010. PMID: 20688520 Free PMC article. Review.
Cited by
-
The role of BST-2/Tetherin in host protection and disease manifestation.Immun Inflamm Dis. 2015 Dec 7;4(1):4-23. doi: 10.1002/iid3.92. eCollection 2016 Mar. Immun Inflamm Dis. 2015. PMID: 27042298 Free PMC article. Review.
-
Structural and functional studies on the extracellular domain of BST2/tetherin in reduced and oxidized conformations.Proc Natl Acad Sci U S A. 2010 Oct 19;107(42):17951-6. doi: 10.1073/pnas.1008206107. Epub 2010 Sep 29. Proc Natl Acad Sci U S A. 2010. PMID: 20880831 Free PMC article.
-
Tetherin is a key effector of the antiretroviral activity of type I interferon in vitro and in vivo.Proc Natl Acad Sci U S A. 2011 Nov 1;108(44):18097-101. doi: 10.1073/pnas.1113694108. Epub 2011 Oct 24. Proc Natl Acad Sci U S A. 2011. PMID: 22025715 Free PMC article.
-
Modulation of HIV-1-host interaction: role of the Vpu accessory protein.Retrovirology. 2010 Dec 22;7:114. doi: 10.1186/1742-4690-7-114. Retrovirology. 2010. PMID: 21176220 Free PMC article. Review.
-
Host restriction factors in retroviral infection: promises in virus-host interaction.Retrovirology. 2012 Dec 20;9:112. doi: 10.1186/1742-4690-9-112. Retrovirology. 2012. PMID: 23254112 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

