Formation of a homocitrate-free iron-molybdenum cluster on NifEN: implications for the role of homocitrate in nitrogenase assembly
- PMID: 20221547
- PMCID: PMC3027845
- DOI: 10.1039/c000264j
Formation of a homocitrate-free iron-molybdenum cluster on NifEN: implications for the role of homocitrate in nitrogenase assembly
Abstract
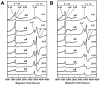
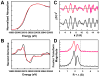
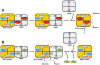
Molybdenum (Mo)-dependent nitrogenase is a complex metalloprotein that catalyzes the biological reduction of dinitrogen (N(2)) to ammonia (NH(3)) at the molybdenum-iron cofactor (FeMoco) site of its molybdenum-iron (MoFe) protein component. Here we report the formation of a homocitrate-free, iron-molybdenum ("FeMo") cluster on the biosynthetic scaffold of FeMoco, NifEN. Such a NifEN-associated "FeMo" cluster exhibits EPR features similar to those of the NifEN-associated, fully-complemented "FeMoco", which originate from the presence of Mo in both cluster species; however, "FeMo" cluster and "FeMoco" display different temperature-dependent changes in the line shape and the signal intensity of their respective EPR features, which reflect the impact of homocitrate on the redox properties of these clusters. XAS/EXAFS analysis reveals that the Mo centers in both "FeMo" cluster and "FeMoco" are present in a similar coordination environment, although Mo in "FeMo" cluster is more loosely coordinated as compared to that in "FeMoco" with respect to the Mo-O distances in the cluster, likely due to the absence of homocitrate that normally serves as an additional ligand for the Mo in the cluster. Subsequent biochemical investigation of the "FeMo" cluster not only facilitates the determination of the sequence of events in the mobilization of Mo and homocitrate during FeMoco maturation, but also permits the examination of the role of homocitrate in the transfer of FeMoco between NifEN and MoFe protein. Combined outcome of these studies establishes a platform for future structural analysis of the interactions between NifEN and MoFe protein, which will provide useful insights into the mechanism of cluster transfer between the two proteins.
Figures


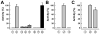
Similar articles
-
Decoding the nitrogenase mechanism: the homologue approach.Acc Chem Res. 2010 Mar 16;43(3):475-84. doi: 10.1021/ar900254x. Acc Chem Res. 2010. PMID: 20030377 Free PMC article.
-
FeMo cofactor maturation on NifEN.Proc Natl Acad Sci U S A. 2006 Nov 14;103(46):17119-24. doi: 10.1073/pnas.0602647103. Epub 2006 Oct 18. Proc Natl Acad Sci U S A. 2006. PMID: 17050696 Free PMC article.
-
Insertion of heterometals into the NifEN-associated iron-molybdenum cofactor precursor.J Biol Inorg Chem. 2010 Mar;15(3):421-8. doi: 10.1007/s00775-009-0614-5. Epub 2009 Dec 5. J Biol Inorg Chem. 2010. PMID: 19967421 Free PMC article.
-
Cluster assembly in nitrogenase.Essays Biochem. 2017 May 9;61(2):271-279. doi: 10.1042/EBC20160071. Print 2017 May 9. Essays Biochem. 2017. PMID: 28487403 Review.
-
Mechanism of Mo-dependent nitrogenase.Annu Rev Biochem. 2009;78:701-22. doi: 10.1146/annurev.biochem.78.070907.103812. Annu Rev Biochem. 2009. PMID: 19489731 Free PMC article. Review.
Cited by
-
Biosynthesis of the metalloclusters of molybdenum nitrogenase.Microbiol Mol Biol Rev. 2011 Dec;75(4):664-77. doi: 10.1128/MMBR.05008-11. Microbiol Mol Biol Rev. 2011. PMID: 22126998 Free PMC article. Review.
-
Biosynthesis of the iron-molybdenum cofactor of nitrogenase.J Biol Chem. 2013 May 10;288(19):13173-7. doi: 10.1074/jbc.R113.454041. Epub 2013 Mar 28. J Biol Chem. 2013. PMID: 23539617 Free PMC article. Review.
-
Nitrogenase assembly.Biochim Biophys Acta. 2013 Aug-Sep;1827(8-9):1112-22. doi: 10.1016/j.bbabio.2012.12.001. Epub 2012 Dec 8. Biochim Biophys Acta. 2013. PMID: 23232096 Free PMC article. Review.
-
Cyanide as a primordial reductant enables a protometabolic reductive glyoxylate pathway.Nat Chem. 2022 Feb;14(2):170-178. doi: 10.1038/s41557-021-00878-w. Epub 2022 Feb 3. Nat Chem. 2022. PMID: 35115655
-
Radical S-adenosyl-L-methionine chemistry in the synthesis of hydrogenase and nitrogenase metal cofactors.J Biol Chem. 2015 Feb 13;290(7):3987-94. doi: 10.1074/jbc.R114.578161. Epub 2014 Dec 4. J Biol Chem. 2015. PMID: 25477518 Free PMC article. Review.
References
-
- Burgess BK, Lowe DJ. Chem Rev. 1996;96:2983. - PubMed
-
- Georgiadis MM, Komiya H, Chakrabarti P, Woo D, Kornuc JJ, Rees DC. Science. 1992;257:1653. - PubMed
-
- Einsle O, Tezcan FA, Andrade SLA, Schmid B, Yoshida M, Howard JB, Rees DC. Science. 2002;297:1696. - PubMed
-
- Schindelin H, Kisker C, Schlessman JL, Howard JB, Rees DC. Nature. 1997;387:370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

