Combined ascorbate and glutathione deficiency leads to decreased cytochrome b5 expression and impaired reduction of sulfamethoxazole hydroxylamine
- PMID: 20221587
- PMCID: PMC2910208
- DOI: 10.1007/s00204-010-0530-z
Combined ascorbate and glutathione deficiency leads to decreased cytochrome b5 expression and impaired reduction of sulfamethoxazole hydroxylamine
Abstract
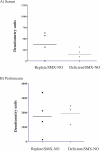
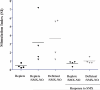
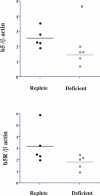
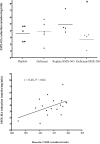
Sulfonamide antimicrobials such as sulfamethoxazole (SMX) have been associated with drug hypersensitivity reactions, particularly in patients with AIDS. A reactive oxidative metabolite, sulfamethoxazole-nitroso (SMX-NO), forms drug-tissue adducts that elicit a T-cell response. Antioxidants such as ascorbic acid (AA) and glutathione (GSH) reduce SMX-NO to the less reactive hydroxylamine metabolite (SMX-HA), which is further reduced to the non-immunogenic parent compound by cytochrome b (5) (b5) and its reductase (b5R). We hypothesized that deficiencies in AA and GSH would enhance drug-tissue adduct formation and immunogenicity toward SMX-NO and that these antioxidant deficiencies might also impair the activity of the b5/b5R pathway. We tested these hypotheses in guinea pigs fed either a normal or AA-restricted diet, followed by buthionine sulfoximine treatment (250 mg/kg SC daily, or vehicle); and SMX-NO (1 mg/kg IP 4 days per week, or vehicle), for 2 weeks. Guinea pigs did not show any biochemical or histopathologic evidence of SMX-NO-related toxicity. Combined AA and GSH deficiency in this model did not significantly increase tissue-drug adduct formation, or splenocyte proliferation in response to SMX-NO. However, combined antioxidant deficiency was associated with decreased mRNA and protein expression of cytochrome b (5), as well as significant decreases in SMX-HA reduction in SMX-NO-treated pigs. These results suggest that SMX-HA detoxification may be down-regulated in combined AA and GSH deficiency. This mechanism could contribute to the higher risk of SMX hypersensitivity in patients with AIDS with antioxidant depletion.
Figures





Similar articles
-
Evaluation of sulfonamide detoxification pathways in haematologic malignancy patients prior to intermittent trimethoprim-sulfamethoxazole prophylaxis.Br J Clin Pharmacol. 2011 Apr;71(4):566-74. doi: 10.1111/j.1365-2125.2010.03889.x. Br J Clin Pharmacol. 2011. PMID: 21204907 Free PMC article.
-
Cytochrome b5 and NADH cytochrome b5 reductase: genotype-phenotype correlations for hydroxylamine reduction.Pharmacogenet Genomics. 2010 Jan;20(1):26-37. doi: 10.1097/FPC.0b013e3283343296. Pharmacogenet Genomics. 2010. PMID: 19997042 Free PMC article.
-
Evaluation of the clinical, immunologic, and biochemical effects of nitroso sulfamethoxazole administration to dogs: a pilot study.Toxicology. 2005 Mar 1;208(1):63-72. doi: 10.1016/j.tox.2004.11.009. Toxicology. 2005. PMID: 15664433
-
Glutathione-ascorbic acid antioxidant system in animals.J Biol Chem. 1994 Apr 1;269(13):9397-400. J Biol Chem. 1994. PMID: 8144521 Review. No abstract available.
-
On the antioxidant effects of ascorbic acid and glutathione.Biochem Pharmacol. 1992 Nov 17;44(10):1905-15. doi: 10.1016/0006-2952(92)90091-v. Biochem Pharmacol. 1992. PMID: 1449510 Review. No abstract available.
Cited by
-
Individual variability in the detoxification of carcinogenic arylhydroxylamines in human breast.Toxicol Sci. 2011 Jun;121(2):245-56. doi: 10.1093/toxsci/kfr073. Epub 2011 Mar 29. Toxicol Sci. 2011. PMID: 21447608 Free PMC article.
-
Antibiotic prophylaxis in immunosuppressed patients - Missed opportunities from trimethoprim-sulfamethoxazole allergy label.World Allergy Organ J. 2024 Jan 3;17(1):100856. doi: 10.1016/j.waojou.2023.100856. eCollection 2024 Jan. World Allergy Organ J. 2024. PMID: 38235260 Free PMC article. Review.
-
Evaluation of sulfonamide detoxification pathways in haematologic malignancy patients prior to intermittent trimethoprim-sulfamethoxazole prophylaxis.Br J Clin Pharmacol. 2011 Apr;71(4):566-74. doi: 10.1111/j.1365-2125.2010.03889.x. Br J Clin Pharmacol. 2011. PMID: 21204907 Free PMC article.
-
Immunogenicity of trimethoprim/sulfamethoxazole in a macaque model of HIV infection.Toxicology. 2016 Aug 10;368-369:10-18. doi: 10.1016/j.tox.2016.08.010. Epub 2016 Aug 23. Toxicology. 2016. PMID: 27565715 Free PMC article.
References
-
- Abramoff MD, Magalhaes PJ, Ram SJ. Image Processing with ImageJ. BIOPHOTONICS INTERNATIONAL. 2004;11:36–43.
-
- Bogden JD, Kemp FW, Han S, Li W, Bruening K, Denny T, Oleske JM, Lloyd J, Baker H, Perez G, Kloser P, Skurnick J, Louria DB. Status of selected nutrients and progression of human immunodeficiency virus type 1 infection. Am J Clin Nutr. 2000;72:809–815. - PubMed
-
- Carr A, Gross AS, Hoskins JM, Penny R, Cooper DA. Acetylation phenotype and cutaneous hypersensitivity to trimethoprim-sulphamethoxazole in HIV-infected patients. Aids. 1994;8:333–337. - PubMed
-
- Cheng L, Stewart BJ, You Q, Petersen DR, Ware JA, Piccotti JR, Kawabata TT, Ju C. Covalent binding of the nitroso metabolite of sulfamethoxazole is important in induction of drug-specific T-cell responses in vivo. Mol Pharmacol. 2008;73:1769–1775. - PubMed
-
- Choi J, Liu RM, Kundu RK, Sangiorgi F, Wu W, Maxson R, Forman HJ. Molecular mechanism of decreased glutathione content in human immunodeficiency virus type 1 Tat-transgenic mice. J Biol Chem. 2000;275:3693–3698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

