Peroxisome-proliferator-activated receptors γ and β/δ mediate vascular endothelial growth factor production in colorectal tumor cells
- PMID: 20221637
- PMCID: PMC11828209
- DOI: 10.1007/s00432-010-0856-1
Peroxisome-proliferator-activated receptors γ and β/δ mediate vascular endothelial growth factor production in colorectal tumor cells
Abstract
Background: Peroxisome-proliferator-activated receptors (PPARs) are nuclear receptors for fatty acids and their derivatives. PPAR subtypes PPARγ and PPARβ/δ are suspected to modulate cancer development in the colon, but their exact role is still discussed controversially.
Methods: The present study investigated the impact of PPARγ and PPARβ/δ on vascular endothelial growth factor (VEGF) and cyclooxygenase 2 (COX-2) expressions induced by synthetic and physiological agonists in the colorectal tumor cell lines SW480 and HT29 using reporter gene assays, qRT-PCR and ELISA.
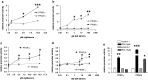
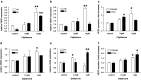
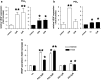
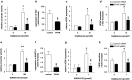
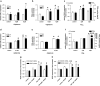
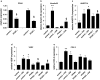
Results: Activation of both PPARγ and PPARβ/δ induced expression of VEGF mRNA and protein in a PPAR-dependent way. The PPARγ agonists ciglitazone and PGJ(2) were the most effective inducers with up to ninefold and threefold increases in VEGF mRNA in SW480 and HT29 cultures, respectively. VEGF secretion was doubled in both cell lines. The PPARβ/δ agonists GW501516 and PGI(2) caused stimulations of only 1.5-fold in both cell lines. In addition, all PPAR agonists induced COX-2 mRNA and secretion of the COX-2 product PGE(2) in HT29 cells. However, this effect was not blocked by knock-down of PPAR expression nor was it essential for VEGF expression as shown by the lack of effect of the COX-2 inhibitor SC236.
Conclusion: In summary, our results identify both PPARγ and PPARβ/δ as an alternative COX-independent mechanism of VEGF induction in colorectal tumor cells.
Figures
Similar articles
-
Peroxisome proliferator-activated receptor (PPAR)-gamma positively controls and PPARalpha negatively controls cyclooxygenase-2 expression in rat brain astrocytes through a convergence on PPARbeta/delta via mutual control of PPAR expression levels.Mol Pharmacol. 2009 Aug;76(2):414-24. doi: 10.1124/mol.109.056010. Epub 2009 May 29. Mol Pharmacol. 2009. PMID: 19483106
-
Editor's Highlight: PPARβ/δ and PPARγ Inhibit Melanoma Tumorigenicity by Modulating Inflammation and Apoptosis.Toxicol Sci. 2017 Oct 1;159(2):436-448. doi: 10.1093/toxsci/kfx147. Toxicol Sci. 2017. PMID: 28962521 Free PMC article.
-
Rosiglitazone induces interleukin-1 receptor antagonist in interleukin-1beta-stimulated rat synovial fibroblasts via a peroxisome proliferator-activated receptor beta/delta-dependent mechanism.Arthritis Rheum. 2005 Mar;52(3):759-69. doi: 10.1002/art.20868. Arthritis Rheum. 2005. PMID: 15751073
-
Peroxisome proliferator-activated receptor gamma agonists for preventing recurrent stroke and other vascular events in people with stroke or transient ischaemic attack.Cochrane Database Syst Rev. 2017 Dec 2;12(12):CD010693. doi: 10.1002/14651858.CD010693.pub4. Cochrane Database Syst Rev. 2017. PMID: 29197071 Free PMC article.
-
Peroxisome proliferator-activated receptor gamma agonists for preventing recurrent stroke and other vascular events in people with stroke or transient ischaemic attack.Cochrane Database Syst Rev. 2023 Jan 10;1(1):CD010693. doi: 10.1002/14651858.CD010693.pub6. Cochrane Database Syst Rev. 2023. PMID: 36625492 Free PMC article.
Cited by
-
PPAR Beta/Delta and the Hallmarks of Cancer.Cells. 2020 May 4;9(5):1133. doi: 10.3390/cells9051133. Cells. 2020. PMID: 32375405 Free PMC article. Review.
-
Dissecting the role of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) in colon, breast, and lung carcinogenesis.Cancer Metastasis Rev. 2011 Dec;30(3-4):619-40. doi: 10.1007/s10555-011-9320-1. Cancer Metastasis Rev. 2011. PMID: 22037942 Free PMC article. Review.
-
β-Catenin and peroxisome proliferator-activated receptor-δ coordinate dynamic chromatin loops for the transcription of vascular endothelial growth factor A gene in colon cancer cells.J Biol Chem. 2012 Nov 30;287(49):41364-73. doi: 10.1074/jbc.M112.377739. Epub 2012 Oct 18. J Biol Chem. 2012. PMID: 23086933 Free PMC article.
-
PPARs Signaling and Cancer in the Gastrointestinal System.PPAR Res. 2012;2012:560846. doi: 10.1155/2012/560846. Epub 2012 Sep 17. PPAR Res. 2012. PMID: 23028383 Free PMC article.
-
Autonomous inhibition of apoptosis correlates with responsiveness of colon carcinoma cell lines to ciglitazone.PLoS One. 2014 Dec 11;9(12):e114158. doi: 10.1371/journal.pone.0114158. eCollection 2014. PLoS One. 2014. PMID: 25502518 Free PMC article.
References
-
- Desvergne B, Ijpenberg A, Devchand PR et al (1998) The peroxisome proliferator-activated receptors at the cross-road of diet and hormonal signalling. J Steroid Biochem Mol Biol 65:65–74 - PubMed
-
- Farrow B, O’Connor KL, Hashimoto K et al (2003) Selective activation of PPARgamma inhibits pancreatic cancer invasion and decreases expression of tissue plasminogen activator. Surgery 134:206–212 - PubMed
-
- Fauconnet S, Lascombe I, Chabannes E et al (2002) Differential regulation of vascular endothelial growth factor expression by peroxisome proliferator-activated receptors in bladder cancer cells. J Biol Chem 277:23534–23543 - PubMed
-
- Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9:669–676 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

