Inducible and constitutive promoters for genetic systems in Sulfolobus acidocaldarius
- PMID: 20221889
- PMCID: PMC2858796
- DOI: 10.1007/s00792-010-0304-9
Inducible and constitutive promoters for genetic systems in Sulfolobus acidocaldarius
Abstract
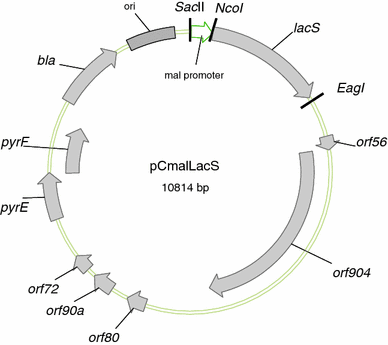
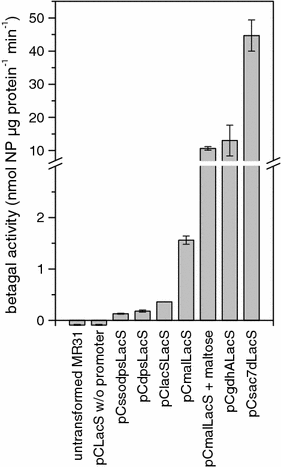
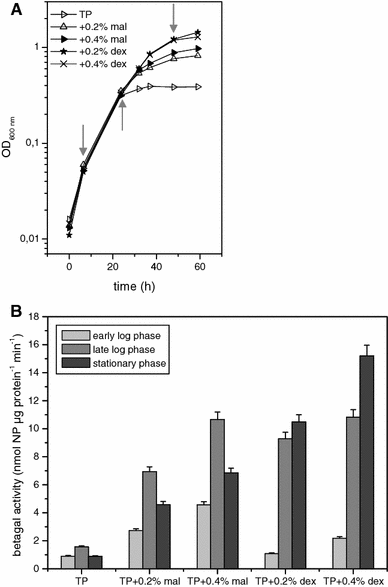
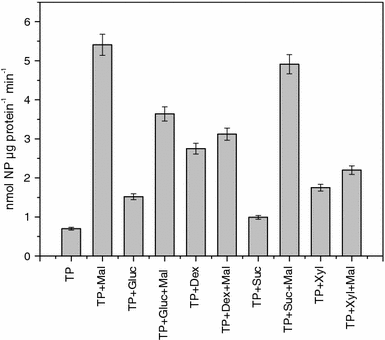
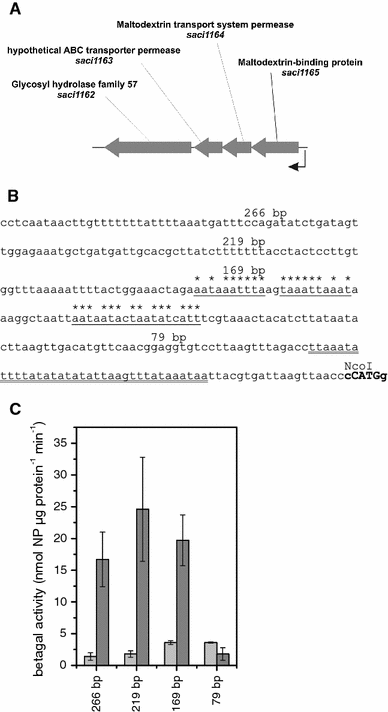
Central to genetic work in any organism are the availability of a range of inducible and constitutive promoters. In this work we studied several promoters for use in the hyperthermophilic archaeon Sulfolobus acidocaldarius. The promoters were tested with the aid of an E. coli-Sulfolobus shuttle vector in reporter gene experiments. As the most suitable inducible promoter a maltose inducible promoter was identified. It comprises 266 bp of the sequence upstream of the gene coding for the maltose/maltotriose binding protein (mbp, Saci_1165). Induction is feasible with either maltose or dextrin at concentrations of 0.2-0.4%. The highest increase in expression (up to 17-fold) was observed in late exponential and stationary phase around 30-50 h after addition of dextrin. Whereas in the presence of glucose and xylose higher basal activity and reduced inducibility with maltose is observed, sucrose can be used in the growth medium additionally without affecting the basal activity or the inducibility. The minimal promoter region necessary could be narrowed down to 169 bp of the upstream sequence. The ABCE1 protein from S. solfataricus was successfully expressed under control of the inducible promoter with the shuttle vector pC and purified from the S. acidocaldarius culture with a yield of about 1 mg L(-1) culture. In addition we also determined the promoter strength of several constitutive promoters.
Figures
References
-
- Barthelme D, Scheele U, Dinkelaker S, Janoschka A, Macmillan F, Albers SV, Driessen AJ, Stagni MS, Bill E, Meyer-Klaucke W, Schunemann V, Tampe R. Structural organization of essential iron-sulfur clusters in the evolutionarily highly conserved ATP-binding cassette protein ABCE1. J Biol Chem. 2007;282:14598–14607. doi: 10.1074/jbc.M700825200. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

