Electrocardiogram signals to assess zebrafish heart regeneration: implication of long QT intervals
- PMID: 20221900
- PMCID: PMC3117900
- DOI: 10.1007/s10439-010-9993-6
Electrocardiogram signals to assess zebrafish heart regeneration: implication of long QT intervals
Abstract
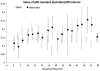
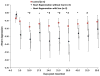
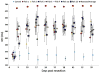
Zebrafish is an emerging model system for cardiac conduction and regeneration. Zebrafish heart regenerates after 20% ventricular resection within 60 days. Whether cardiac conduction phenotype correlated with cardiomyocyte regeneration remained undefined. Longitudinal monitoring of the adult zebrafish heart (n = 12) was performed in terms of atrial contraction (PR intervals), ventricular depolarization (QRS complex) and repolarization (heart rated corrected QTc interval). Baseline electrocardiogram (ECG) signals were recorded one day prior to resection and twice per week over 59 days. Immunostaining for gap junctions with anti-Connexin-43 antibody was compared between the sham (n = 5) and ventricular resection at 60 days post-resection (dpr) (n = 7). Heart rate variability, QTc prolongation and J-point depression developed in the resected group but not in the sham. Despite a trend toward heart rate variability in response to ventricular resection, the differences between the resected and sham fish were, by and large, statistically insignificant. At 10 dpr, J-point depression was statistically significant (sham: -0.179 +/- 0.061 mV vs. ventricular resection: -0.353 +/- 0.105 mV, p < 0.01, n = 7). At 60 days, histology revealed either cardiomyocyte regeneration (n = 4) or scar tissues (n = 3). J-point depression was no longer statistically significant at 59 dpr (sham: -0.114 +/- 0.085 mV; scar tissue: -0.268 +/- 0.178 mV, p > 0.05, n = 3; regeneration: -0.209 +/- 0.119 mV, p > 0.05, n = 4). Despite positive Connexin-43 staining in the regeneration group, QTc intervals remained prolonged (sham: 325 +/- 42 ms, n = 5; scar tissues: 534 +/- 51 ms, p < 0.01, n = 3; regeneration: 496 +/- 31 ms, p < 0.01, n = 4). Thus, we observed delayed electric repolarization in either the regenerated hearts or scar tissues. Moreover, early regenerated cardiomyocytes lacked the conduction phenotypes of the sham fish.
Figures


Similar articles
-
Micro-electrocardiograms to study post-ventricular amputation of zebrafish heart.Ann Biomed Eng. 2009 May;37(5):890-901. doi: 10.1007/s10439-009-9668-3. Epub 2009 Mar 12. Ann Biomed Eng. 2009. PMID: 19280341 Free PMC article.
-
Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes.Nature. 2010 Mar 25;464(7288):601-5. doi: 10.1038/nature08804. Nature. 2010. PMID: 20336144 Free PMC article.
-
Flexible microelectrode arrays to interface epicardial electrical signals with intracardial calcium transients in zebrafish hearts.Biomed Microdevices. 2012 Apr;14(2):357-66. doi: 10.1007/s10544-011-9612-9. Biomed Microdevices. 2012. PMID: 22124886 Free PMC article.
-
A Systematic Exposition of Methods used for Quantification of Heart Regeneration after Apex Resection in Zebrafish.Cells. 2020 Feb 26;9(3):548. doi: 10.3390/cells9030548. Cells. 2020. PMID: 32111059 Free PMC article.
-
Assessment of QTc and Risk of Torsades de Pointes in Ventricular Conduction Delay and Pacing: A Review of the Literature and Call to Action.J Acad Consult Liaison Psychiatry. 2021 Sep-Oct;62(5):501-510. doi: 10.1016/j.jaclp.2021.02.003. Epub 2021 Feb 13. J Acad Consult Liaison Psychiatry. 2021. PMID: 34489062 Review.
Cited by
-
Consecutive treatments of methamphetamine promote the development of cardiac pathological symptoms in zebrafish.PLoS One. 2023 Nov 17;18(11):e0294322. doi: 10.1371/journal.pone.0294322. eCollection 2023. PLoS One. 2023. PMID: 37976248 Free PMC article.
-
Endothelial mechanotransduction in cardiovascular development and regeneration: emerging approaches and animal models.Curr Top Membr. 2021;87:131-151. doi: 10.1016/bs.ctm.2021.07.002. Epub 2021 Oct 12. Curr Top Membr. 2021. PMID: 34696883 Free PMC article. Review.
-
Quantitative 4D imaging of biomechanical regulation of ventricular growth and maturation.Curr Opin Biomed Eng. 2023 Jun;26:100438. doi: 10.1016/j.cobme.2022.100438. Epub 2022 Dec 21. Curr Opin Biomed Eng. 2023. PMID: 37424697 Free PMC article.
-
Imaging escape and avoidance behavior in zebrafish larvae.Rev Neurosci. 2011;22(1):63-73. doi: 10.1515/RNS.2011.008. Rev Neurosci. 2011. PMID: 21572576 Free PMC article.
-
Use of echocardiography reveals reestablishment of ventricular pumping efficiency and partial ventricular wall motion recovery upon ventricular cryoinjury in the zebrafish.PLoS One. 2014 Dec 22;9(12):e115604. doi: 10.1371/journal.pone.0115604. eCollection 2014. PLoS One. 2014. PMID: 25532015 Free PMC article.
References
-
- Braunwald E, Zipes DP, Libby P. Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia, PA: W.B. Saunders Company; 2001.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

