Compartmental transport model of microbicide delivery by an intravaginal ring
- PMID: 20222027
- PMCID: PMC3072789
- DOI: 10.1002/jps.22120
Compartmental transport model of microbicide delivery by an intravaginal ring
Abstract
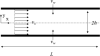
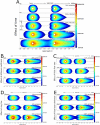
Topical antimicrobials, or microbicides, are being developed to prevent HIV transmission through local, mucosal delivery of antiviral compounds. While hydrogel vehicles deliver the majority of current microbicide products, intravaginal rings (IVRs) are an alternative microbicide modality in preclinical development. IVRs provide a long-term dosing alternative to hydrogel use, and might provide improved user adherence. IVR efficacy requires sustained delivery of antiviral compounds to the entire vaginal compartment. A two-dimensional, compartmental vaginal drug transport model was created to evaluate the delivery of drugs from an intravaginal ring. The model utilized MRI-derived ring geometry and location, experimentally defined ring fluxes and vaginal fluid velocities, and biophysically relevant transport theory. Model outputs indicated the presence of potentially inhibitory concentrations of antiviral compounds along the entire vaginal canal within 24 h following IVR insertion. Distributions of inhibitory concentrations of antiviral compounds were substantially influenced by vaginal fluid flow and production, while showing little change due to changes in diffusion coefficients or ring fluxes. Additionally, model results were predictive of in vivo concentrations obtained in clinical trials. Overall, this analysis initiates a mechanistic computational framework, heretofore missing, to understand and evaluate the potential of IVRs for effective delivery of antiviral compounds.
(c) 2010 Wiley-Liss, Inc. and the American Pharmacists Association
Figures



Similar articles
-
Vaginal deployment and tenofovir delivery by microbicide gels.Drug Deliv Transl Res. 2015 Jun;5(3):279-94. doi: 10.1007/s13346-015-0227-1. Drug Deliv Transl Res. 2015. PMID: 25874971 Free PMC article.
-
Intravaginal ring delivery of the reverse transcriptase inhibitor TMC 120 as an HIV microbicide.Int J Pharm. 2006 Nov 15;325(1-2):82-9. doi: 10.1016/j.ijpharm.2006.06.026. Epub 2006 Jun 23. Int J Pharm. 2006. PMID: 16884869
-
Phase I trial of pod-intravaginal rings delivering antiretroviral agents for HIV-1 prevention: Rectal drug exposure from vaginal dosing with tenofovir disoproxil fumarate, emtricitabine, and maraviroc.PLoS One. 2018 Aug 22;13(8):e0201952. doi: 10.1371/journal.pone.0201952. eCollection 2018. PLoS One. 2018. PMID: 30133534 Free PMC article. Clinical Trial.
-
Intravaginal rings as delivery systems for microbicides and multipurpose prevention technologies.Int J Womens Health. 2013 Oct 21;5:695-708. doi: 10.2147/IJWH.S34030. Int J Womens Health. 2013. PMID: 24174884 Free PMC article. Review.
-
Advances in microbicide vaginal rings.Antiviral Res. 2010 Dec;88 Suppl 1:S30-9. doi: 10.1016/j.antiviral.2010.09.003. Antiviral Res. 2010. PMID: 21109066 Review.
Cited by
-
An intravaginal ring that releases the NNRTI MIV-150 reduces SHIV transmission in macaques.Sci Transl Med. 2012 Sep 5;4(150):150ra123. doi: 10.1126/scitranslmed.3003936. Sci Transl Med. 2012. PMID: 22956201 Free PMC article.
-
Intravaginal rings: controlled release systems for contraception and prevention of transmission of sexually transmitted infections.Drug Deliv Transl Res. 2011 Jun;1(3):185-93. doi: 10.1007/s13346-011-0024-4. Drug Deliv Transl Res. 2011. PMID: 25788239
-
Physiologically-based pharmacokinetic model of vaginally administered dapivirine ring and film formulations.Br J Clin Pharmacol. 2018 Sep;84(9):1950-1969. doi: 10.1111/bcp.13625. Epub 2018 Jun 19. Br J Clin Pharmacol. 2018. PMID: 29714824 Free PMC article.
-
Preformulation studies of EFdA, a novel nucleoside reverse transcriptase inhibitor for HIV prevention.Drug Dev Ind Pharm. 2014 Aug;40(8):1101-11. doi: 10.3109/03639045.2013.809535. Epub 2013 Jul 10. Drug Dev Ind Pharm. 2014. PMID: 23841536 Free PMC article.
-
Vaginal drug distribution modeling.Adv Drug Deliv Rev. 2015 Sep 15;92:2-13. doi: 10.1016/j.addr.2015.04.017. Epub 2015 Apr 28. Adv Drug Deliv Rev. 2015. PMID: 25933938 Free PMC article. Review.
References
-
- Fichorova RN, Tucker LD, Anderson DJ. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. Journal Of Infectious Diseases. 2001;184(4):418–428. - PubMed
-
- Lederman MM, Offord RE, Hartley O. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nature Reviews Immunology. 2006;6(5):371–382. - PubMed
-
- Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nature Reviews Immunology. 2005;5(10):783–792. - PubMed
-
- Stone A. Microbicides: A new approach to preventing HIV and other sexually transmitted infections. Nature Reviews Drug Discovery. 2002;1(12):977–985. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

