Total synthesis and configurational assignment of chondramide A
- PMID: 20222097
- PMCID: PMC2899681
- DOI: 10.1002/chem.200903500
Total synthesis and configurational assignment of chondramide A
Abstract
The first total synthesis of the cyclodepsipeptide chondramide A (2 b) is described. This depsipeptide is composed of four subunits, namely L-alanine, N-Me-D-tryptophan, 3-amino-2-methoxy-propionic acid (beta-tyrosine derivative), and a 7-hydroxy-alkenoic acid. While the configuration of the stereogenic centers in the 7-hydroxy-alkenoic acid were known, the configuration of the tyrosine derivative required clarification and turned out to be (2S,3R) or (2L,3L), respectively. The synthesis of the 3-amino-2-methoxy-3-arylpropanoic ester 20 b relied on an asymmetric dihydroxylation yielding diol ent-15 a followed by a regioselective Mitsunobu substitution leading to 3-azido-2-hydroxypropanoate 18 b. We could also show that the ester bond in the seco compound 26 b can be fashioned by a Mitsunobu esterification by using hydroxy ester (7S)-7 and the tripeptide acid 25 b. This synthesis should allow for the preparation of various analogues.
Figures








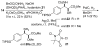


Similar articles
-
Synthesis of chondramide A analogues with modified β-tyrosine and their biological evaluation.Chemistry. 2011 Nov 18;17(47):13349-57. doi: 10.1002/chem.201101978. Epub 2011 Oct 20. Chemistry. 2011. PMID: 22012705 Free PMC article.
-
Chondramide C: synthesis, configurational assignment, and structure-activity relationship studies.Angew Chem Int Ed Engl. 2008;47(34):6478-82. doi: 10.1002/anie.200801156. Angew Chem Int Ed Engl. 2008. PMID: 18624309 No abstract available.
-
Efficient and versatile stereoselective synthesis of cryptophycins.Chemistry. 2005 Aug 5;11(16):4667-77. doi: 10.1002/chem.200500282. Chemistry. 2005. PMID: 15915529
-
[Total Synthesis of Biologically Active Natural Products toward Elucidation of the Mode of Action].Yakugaku Zasshi. 2015;135(10):1099-108. doi: 10.1248/yakushi.15-00184. Yakugaku Zasshi. 2015. PMID: 26423864 Review. Japanese.
-
Biosynthesis of depsipeptides, or Depsi: The peptides with varied generations.Protein Sci. 2020 Dec;29(12):2316-2347. doi: 10.1002/pro.3979. Epub 2020 Nov 2. Protein Sci. 2020. PMID: 33073901 Free PMC article. Review.
Cited by
-
Biostructural features of additional jasplakinolide (jaspamide) analogues.J Nat Prod. 2011 Mar 25;74(3):341-51. doi: 10.1021/np100721g. Epub 2011 Jan 11. J Nat Prod. 2011. PMID: 21241058 Free PMC article.
-
Synthesis of the polyketide section of seragamide A and related cyclodepsipeptides via Negishi cross coupling.Beilstein J Org Chem. 2019 Feb 28;15:577-583. doi: 10.3762/bjoc.15.53. eCollection 2019. Beilstein J Org Chem. 2019. PMID: 30873243 Free PMC article.
-
Synthetic chondramide A analogues stabilize filamentous actin and block invasion by Toxoplasma gondii.J Nat Prod. 2013 Sep 27;76(9):1565-72. doi: 10.1021/np400196w. Epub 2013 Sep 10. J Nat Prod. 2013. PMID: 24020843 Free PMC article.
-
Modulation of actin dynamics as potential macrophage subtype-targeting anti-tumour strategy.Sci Rep. 2017 Jan 30;7:41434. doi: 10.1038/srep41434. Sci Rep. 2017. PMID: 28134280 Free PMC article.
-
Synthesis of chondramide A analogues with modified β-tyrosine and their biological evaluation.Chemistry. 2011 Nov 18;17(47):13349-57. doi: 10.1002/chem.201101978. Epub 2011 Oct 20. Chemistry. 2011. PMID: 22012705 Free PMC article.
References
-
-
For recent reviews, see: Myles DC. Ann Rep Med Chem. 2002;37:125–132.Altmann KH, Gertsch J. Nat Prod Rep. 2007;24:327–357.Altmann KH, Pfeiffer B, Arseniyadis S, Pratt BA, Nicolaou KC. Chem Med Chem. 2007;2:396–423.Nicolaou KC, Chen JS, Dalby SM. Bioorg Med Chem. 2009;17:2290–2303.
-
-
-
For review about cyclodepsipeptides, see: Hamada Y, Shioiri T. Chem Rev. 2005;105:4441–4482.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

