The involvement of human RECQL4 in DNA double-strand break repair
- PMID: 20222902
- PMCID: PMC4624395
- DOI: 10.1111/j.1474-9726.2010.00562.x
The involvement of human RECQL4 in DNA double-strand break repair
Abstract
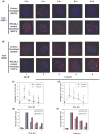
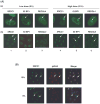
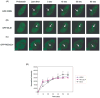
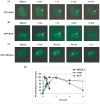
Rothmund-Thomson syndrome (RTS) is an autosomal recessive hereditary disorder associated with mutation in RECQL4 gene, a member of the human RecQ helicases. The disease is characterized by genomic instability, skeletal abnormalities and predisposition to malignant tumors, especially osteosarcomas. The precise role of RECQL4 in cellular pathways is largely unknown; however, recent evidence suggests its involvement in multiple DNA metabolic pathways. This study investigates the roles of RECQL4 in DNA double-strand break (DSB) repair. The results show that RECQL4-deficient fibroblasts are moderately sensitive to gamma-irradiation and accumulate more gammaH2AX and 53BP1 foci than control fibroblasts. This is suggestive of defects in efficient repair of DSB's in the RECQL4-deficient fibroblasts. Real time imaging of live cells using laser confocal microscopy shows that RECQL4 is recruited early to laser-induced DSBs and remains for a shorter duration than WRN and BLM, indicating its distinct role in repair of DSBs. Endogenous RECQL4 also colocalizes with gammaH2AX at the site of DSBs. The RECQL4 domain responsible for its DNA damage localization has been mapped to the unique N-terminus domain between amino acids 363-492, which shares no homology to recruitment domains of WRN and BLM to the DSBs. Further, the recruitment of RECQL4 to laser-induced DNA damage is independent of functional WRN, BLM or ATM proteins. These results suggest distinct cellular dynamics for RECQL4 protein at the site of laser-induced DSB and that it might play important roles in efficient repair of DSB's.
Figures




Similar articles
-
Recruitment and retention dynamics of RECQL5 at DNA double strand break sites.DNA Repair (Amst). 2012 Jul 1;11(7):624-35. doi: 10.1016/j.dnarep.2012.05.001. Epub 2012 May 24. DNA Repair (Amst). 2012. PMID: 22633600 Free PMC article.
-
Sensitivity of RECQL4-deficient fibroblasts from Rothmund-Thomson syndrome patients to genotoxic agents.Hum Genet. 2008 Jul;123(6):643-53. doi: 10.1007/s00439-008-0518-4. Epub 2008 May 27. Hum Genet. 2008. PMID: 18504617 Free PMC article.
-
Direct and indirect roles of RECQL4 in modulating base excision repair capacity.Hum Mol Genet. 2009 Sep 15;18(18):3470-83. doi: 10.1093/hmg/ddp291. Epub 2009 Jun 29. Hum Mol Genet. 2009. PMID: 19567405 Free PMC article.
-
Human RecQL4 helicase plays multifaceted roles in the genomic stability of normal and cancer cells.Cancer Lett. 2018 Jan 28;413:1-10. doi: 10.1016/j.canlet.2017.10.021. Epub 2017 Nov 7. Cancer Lett. 2018. PMID: 29080750 Review.
-
The molecular role of the Rothmund-Thomson-, RAPADILINO- and Baller-Gerold-gene product, RECQL4: recent progress.Cell Mol Life Sci. 2007 Apr;64(7-8):796-802. doi: 10.1007/s00018-007-6468-5. Cell Mol Life Sci. 2007. PMID: 17364146 Free PMC article. Review.
Cited by
-
Human RecQ Helicases in DNA Double-Strand Break Repair.Front Cell Dev Biol. 2021 Feb 25;9:640755. doi: 10.3389/fcell.2021.640755. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33718381 Free PMC article. Review.
-
RECQ helicase RECQL4 participates in non-homologous end joining and interacts with the Ku complex.Carcinogenesis. 2014 Nov;35(11):2415-24. doi: 10.1093/carcin/bgu137. Epub 2014 Jun 18. Carcinogenesis. 2014. PMID: 24942867 Free PMC article.
-
The DNA helicase recql4 is required for normal osteoblast expansion and osteosarcoma formation.PLoS Genet. 2015 Apr 10;11(4):e1005160. doi: 10.1371/journal.pgen.1005160. eCollection 2015 Apr. PLoS Genet. 2015. PMID: 25859855 Free PMC article.
-
Molecular Mechanisms of the RECQ4 Pathogenic Mutations.Front Mol Biosci. 2021 Nov 18;8:791194. doi: 10.3389/fmolb.2021.791194. eCollection 2021. Front Mol Biosci. 2021. PMID: 34869606 Free PMC article. Review.
-
Meta-analysis of DNA double-strand break response kinetics.Nucleic Acids Res. 2017 Dec 15;45(22):12625-12637. doi: 10.1093/nar/gkx1128. Nucleic Acids Res. 2017. PMID: 29182755 Free PMC article.
References
-
- Anbari KK, Ierardi-Curto LA, Silber JS, Asada N, Spinner N, Zackai EH, Belasco J, Morrissette JD, Dormans JP. Two primary osteosarcomas in a patient with Rothmund-Thomson syndrome. Clinical Orthopaedics and Related Research. 2000;378:213–223. - PubMed
-
- Burks LM, Yin JH, Plon SE. Nuclear import and retention domains in the amino terminus of RECQL4. Gene. 2007;391:26–38. - PubMed
-
- Cabral RE, Queille S, Bodemer C, de PY, Neto JB, Sarasin A, ya-Grosjean L. Identification of new RECQL4 mutations in Caucasian Rothmund-Thomson patients and analysis of sensitivity to a wide range of genotoxic agents. Mutat Res. 2008;643:41–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

