Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex
- PMID: 20223206
- PMCID: PMC2849994
- DOI: 10.1016/j.neuron.2010.02.019
Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex
Abstract
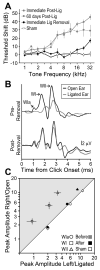
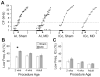
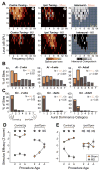
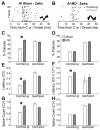
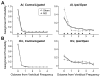
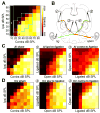
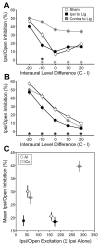
Degraded sensory experience during critical periods of development can have adverse effects on brain function. In the auditory system, conductive hearing loss associated with childhood ear infections can produce long-lasting deficits in auditory perceptual acuity, much like amblyopia in the visual system. Here we explore the neural mechanisms that may underlie "amblyaudio" by inducing reversible monaural deprivation (MD) in infant, juvenile, and adult rats. MD distorted tonotopic maps, weakened the deprived ear's representation, strengthened the open ear's representation, and disrupted binaural integration of interaural level differences (ILD). Bidirectional plasticity effects were strictly governed by critical periods, were more strongly expressed in primary auditory cortex than inferior colliculus, and directly impacted neural coding accuracy. These findings highlight a remarkable degree of competitive plasticity between aural representations and suggest that the enduring perceptual sequelae of childhood hearing loss might be traced to maladaptive plasticity during critical periods of auditory cortex development.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Monaural Congenital Deafness Affects Aural Dominance and Degrades Binaural Processing.Cereb Cortex. 2016 Apr;26(4):1762-77. doi: 10.1093/cercor/bhv351. Epub 2016 Jan 22. Cereb Cortex. 2016. PMID: 26803166 Free PMC article.
-
Effects of ear plugging on single-unit azimuth sensitivity in cat primary auditory cortex. II. Azimuth tuning dependent upon binaural stimulation.J Neurophysiol. 1994 Jun;71(6):2194-216. doi: 10.1152/jn.1994.71.6.2194. J Neurophysiol. 1994. PMID: 7931511
-
Sustained Perceptual Deficits from Transient Sensory Deprivation.J Neurosci. 2015 Jul 29;35(30):10831-42. doi: 10.1523/JNEUROSCI.0837-15.2015. J Neurosci. 2015. PMID: 26224865 Free PMC article.
-
Anatomy and physiology of binaural hearing.Audiology. 1991;30(3):125-34. doi: 10.3109/00206099109072878. Audiology. 1991. PMID: 1953442 Review.
-
Central plasticity and dysfunction elicited by aural deprivation in the critical period.Front Neural Circuits. 2015 Jun 2;9:26. doi: 10.3389/fncir.2015.00026. eCollection 2015. Front Neural Circuits. 2015. PMID: 26082685 Free PMC article. Review.
Cited by
-
Differential maturation of vesicular glutamate and GABA transporter expression in the mouse auditory forebrain during the first weeks of hearing.Brain Struct Funct. 2016 Jun;221(5):2619-73. doi: 10.1007/s00429-015-1062-3. Epub 2015 Jul 10. Brain Struct Funct. 2016. PMID: 26159773 Free PMC article.
-
Auditory midbrain coding of statistical learning that results from discontinuous sensory stimulation.PLoS Biol. 2018 Jul 26;16(7):e2005114. doi: 10.1371/journal.pbio.2005114. eCollection 2018 Jul. PLoS Biol. 2018. PMID: 30048446 Free PMC article.
-
Duration of unilateral auditory deprivation is associated with reduced speech perception after cochlear implantation: A single-sided deafness study.Cochlear Implants Int. 2019 Mar;20(2):51-56. doi: 10.1080/14670100.2018.1550469. Epub 2018 Nov 28. Cochlear Implants Int. 2019. PMID: 30486762 Free PMC article.
-
NCS Assessments of the Motor, Sensory, and Physical Health Domains.Front Pediatr. 2021 Nov 26;9:622542. doi: 10.3389/fped.2021.622542. eCollection 2021. Front Pediatr. 2021. PMID: 34900852 Free PMC article.
-
Chronic Unilateral Hearing Loss Disrupts Neural Tuning to Sound-Source Azimuth in the Rat Primary Auditory Cortex.Front Neurosci. 2019 May 10;13:477. doi: 10.3389/fnins.2019.00477. eCollection 2019. Front Neurosci. 2019. PMID: 31133797 Free PMC article.
References
-
- Bendor D, Wang X. Differential neural coding of acoustic flutter within primate auditory cortex. Nat Neurosci. 2007;10:763–771. - PubMed
-
- Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300:498–502. - PubMed
-
- Clements M, Kelly JB. Auditory spatial responses of young guinea pigs (Cavia porcellus) during and after ear blocking. J Comp Physiol Psychol. 1978;92:34–44. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

