Isopropylamine NONOate (IPA/NO) moderates neointimal hyperplasia following vascular injury
- PMID: 20223627
- PMCID: PMC2860688
- DOI: 10.1016/j.jvs.2009.12.028
Isopropylamine NONOate (IPA/NO) moderates neointimal hyperplasia following vascular injury
Abstract
Objective: Isopropylamine NONOate (IPA/NO) is a nitroxyl (HNO) donor at physiologic pH. HNO is a positive inotrope and vasodilator, but little is known about its effect on neointimal hyperplasia. The aims of this study are to determine the effect of IPA/NO on endothelial and vascular smooth muscle cells (VSMC) in vitro and to determine if IPA/NO inhibits neointimal hyperplasia in vivo.
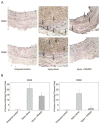
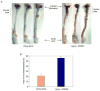
Methods: VSMC were harvested from the abdominal aortas of male Sprague Dawley rats, and human umbilical vein endothelial cells were purchased from ATCC. In vitro, cellular proliferation was assessed by (3)H-thymidine incorporation, cell migration was assessed using the scrape assay, and cell death was assessed using Guava personal cell analysis (PCA). Cell cycle analysis was performed using propidium iodide staining and flow cytometry analysis. Protein expression was assessed using Western blot analysis. Phosphorylated proteins were assessed using immunoprecipitation and Western blot analysis. In vivo, the carotid artery injury model was performed on male Sprague Dawley rats treated with (n = 12) or without (n = 6) periadventitial IPA/NO (10 mg). Arteries harvested at 2 weeks were assessed for morphometrics using ImageJ. Inflammation was assessed using immunohistochemistry. Endothelialization was assessed by Evans blue staining of carotid arteries harvested 7 days after balloon injury from rats treated with (n = 6) or without (n = 3) periadventitial IPA/NO (10 mg).
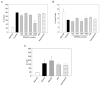
Results: In vitro, 1000 micromol/L IPA/NO inhibited both VSMC (38.7 +/- 4.5% inhibition vs control, P = .003) and endothelial cell proliferation (54.0 +/- 2.9% inhibition vs control, P < or = 0.001) without inducing cell death or inhibiting migration. In VSMC, this inhibition was associated with an S-phase cell cycle arrest and increased expression of cyclin A, cyclin D1, and the cyclin-dependent kinase inhibitor p21. No change was noted in the phosphorylation status of cdk2, cdk4, or cdk6 by IPA/NO. In rodents subjected to the carotid artery balloon injury model, IPA/NO caused significant reductions in neointimal area (298 +/- 20 vs 422 +/- 30, P < or = .001) and medial area (311 +/- 14 vs 449 +/- 16, P < or = .001) compared with injury alone, and reduced macrophage infiltration to 1.7 +/- 0.8 from 16.1 +/- 3.5 cells per high power field (P < or = .001). IPA/NO also prevented re-endothelialization compared with injury alone (55.9 +/- 0.5% nonendothelialized vs 21 +/- 4.4%, respectively, P = .001). Lastly, a 50% mortality rate was observed in the IPA/NO-treated groups.
Conclusions: In summary, while IPA/NO modestly inhibited neointimal hyperplasia by inhibiting VSMC proliferation and macrophage infiltration, it also inhibited endothelial cell proliferation and induced significant mortality in our animal model. Since HNO is being investigated as a treatment for congestive heart failure, our results raise some concerns about the use of IPA/NO in the vasculature and suggest that further studies be conducted on the safety of HNO donors in the cardiovascular system.
Published by Mosby, Inc.
Figures




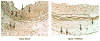
Similar articles
-
Nitric oxide and nanotechnology: a novel approach to inhibit neointimal hyperplasia.J Vasc Surg. 2008 Jan;47(1):173-82. doi: 10.1016/j.jvs.2007.09.005. J Vasc Surg. 2008. PMID: 18178471 Free PMC article.
-
Nitric oxide inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia by increasing the ubiquitination and degradation of UbcH10.Cell Biochem Biophys. 2011 Jun;60(1-2):89-97. doi: 10.1007/s12013-011-9179-3. Cell Biochem Biophys. 2011. PMID: 21448667 Free PMC article.
-
N-oleoylethanolamide suppresses intimal hyperplasia after balloon injury in rats through AMPK/PPARα pathway.Biochem Biophys Res Commun. 2018 Feb 5;496(2):415-421. doi: 10.1016/j.bbrc.2018.01.015. Epub 2018 Jan 4. Biochem Biophys Res Commun. 2018. PMID: 29305859
-
Cinnamic aldehyde inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia in Zucker Diabetic Fatty rats.Redox Biol. 2018 Oct;19:166-178. doi: 10.1016/j.redox.2018.08.013. Epub 2018 Aug 24. Redox Biol. 2018. PMID: 30172101 Free PMC article.
-
Intimal hyperplasia in murine models.Curr Drug Targets. 2008 Mar;9(3):251-60. doi: 10.2174/138945008783755601. Curr Drug Targets. 2008. PMID: 18336244 Free PMC article. Review.
Cited by
-
Coating small-diameter ePTFE vascular grafts with tunable poly(diol-co-citrate-co-ascorbate) elastomers to reduce neointimal hyperplasia.Biomater Sci. 2021 Aug 7;9(15):5160-5174. doi: 10.1039/d1bm00101a. Epub 2021 Apr 6. Biomater Sci. 2021. PMID: 34312627 Free PMC article.
-
Antioxidants modulate the antiproliferative effects of nitric oxide on vascular smooth muscle cells and adventitial fibroblasts by regulating oxidative stress.Am J Surg. 2011 Nov;202(5):536-40. doi: 10.1016/j.amjsurg.2011.06.018. Epub 2011 Sep 23. Am J Surg. 2011. PMID: 21944289 Free PMC article.
-
Therapeutic Potential of Nitroxyl (HNO) Donors in the Management of Acute Decompensated Heart Failure.Drugs. 2016 Sep;76(14):1337-48. doi: 10.1007/s40265-016-0631-y. Drugs. 2016. PMID: 27566478 Review.
-
Development and Characterization of Chitosan and Chondroitin Sulfate Based Hydrogels Enriched with Garlic Extract for Potential Wound Healing/Skin Regeneration Applications.Gels. 2022 Oct 20;8(10):676. doi: 10.3390/gels8100676. Gels. 2022. PMID: 36286177 Free PMC article.
-
Recent Developments in Pharmacological Effect, Mechanism and Application Prospect of Diazeniumdiolates.Front Pharmacol. 2020 Jun 23;11:923. doi: 10.3389/fphar.2020.00923. eCollection 2020. Front Pharmacol. 2020. PMID: 32655397 Free PMC article. Review.
References
-
- Drago RS, Karstetter BR. The reaction of nitrogen(II) oxide with various primary and secondary amines. J Am Chem Soc. 1961;83:1819–22.
-
- Fukuto JM, Bartberger MD, Dutton AS, Paolocci N, Wink DA, Houk KN. The physiological chemistry and biological activity of nitroxyl (HNO): the neglected, misunderstood, and enigmatic nitrogen oxide. Chem Res Toxicol. 2005 May;18(5):790–801. - PubMed
-
- Dutton AS, Suhrada CP, Miranda KM, Wink DA, Fukuto JM, Houk KN. Mechanism of pH-dependent decomposition of monoalkylamine diazeniumdiolates to form HNO and NO, deduced from the model compound methylamine diazeniumdiolate, density functional theory, and CBS-QB3 calculations. Inorg Chem. 2006 Mar 20;45(6):2448–56. - PMC - PubMed
-
- Bonner FT, Ravid B. Thermal decomposition of oxyhyponitrite (sodium trioxodinitrate(II)) in aqueous solution. Inorg Chem. 1975;14:558–63.
-
- Hughes MN, Wimbledon PE. The chemistry of trioxodinitrates. 1. Decomposition of sodium trioxdinitrate (Angeli’s salt) in aqueous solution. J Chem Soc Dalton Trans. 1976;8:703–7.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

