Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity
- PMID: 20223680
- PMCID: PMC2880185
- DOI: 10.1016/j.tem.2010.01.009
Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity
Abstract
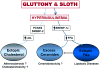
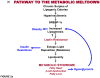
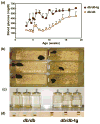
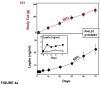
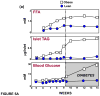
Once considered divine retribution for sins, comorbidities of obesity (metabolic syndrome) are today attributed to obesity-induced metabolic defects. Here, we propose that obesity and hyperleptinemia protect lipid-intolerant nonadipose organs against lipotoxic lipid spillover during sustained caloric surplus. Metabolic syndrome is ascribed to lipotoxicity caused by age-related resistance to antilipotoxic protection by leptin.
Published by Elsevier Ltd.
Figures



References
-
- Cohen J, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161–165. - PubMed
-
- Keys A. Human atherosclerosis and the diet. Circulation. 1952;5:115–118. - PubMed
-
- Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:319–336. - PubMed
-
- Unger RH. Lipid overload and overflow: metabolic trauma and the metabolic syndrome. Trends Endocrinol Metab. 2003;14:398–403. - PubMed
-
- Unger RH. The physiology of cellular liporegulation. Annu Rev Physiol. 2003;65:333–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

