How important is the phosphatase activity of sensor kinases?
- PMID: 20223700
- PMCID: PMC2853374
- DOI: 10.1016/j.mib.2010.01.013
How important is the phosphatase activity of sensor kinases?
Abstract
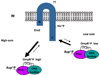
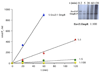
In two-component signaling systems, phosphorylated response regulators (RRs) are often dephosphorylated by their partner kinases in order to control the in vivo concentration of phospho-RR (RR approximately P). This activity is easily demonstrated in vitro, but these experiments have typically used very high concentrations of the histidine kinase (HK) compared to the RR approximately P. Many two-component systems exhibit exquisite control over the ratio of HK to RR in vivo. The question thus arises as to whether the phosphatase activity of HKs is significant in vivo. This topic will be explored in the present review.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures


References
-
- Tanaka T, Saha SK, Tomomori C, Ishima R, Liu D, Tong KI, Park H, Dutta R, Qin L, Swindells MB, et al. NMR structure of the histidine kinase domain of the E. coli osmosensor EnvZ. Nature. 1998;396:88–92. - PubMed
-
- Tomomori C, Tanaka T, Dutta R, Park H, Saha SK, Zhu Y, Ishima R, Liu D, Tong KI, Kurokawa H, et al. Solution structure of the homodimeric core domain of Escherichia coli histidine kinase EnvZ. Nat Struct Biol. 1999;6:729–734. - PubMed
-
-
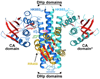
Casino P, Rubio V, Marina A. Structural insight into partner specificity and phosphoryl transfer in two-component signal transduction. Cell. 2009;139:325–336. ••This is a co-crystal structure of an HK complexed with its RR from T. maritima. The authors make a strong case for HK phosphorylation occurring in cis rather than trans.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

