Mycobacterium tuberculosis origin of replication and the promoter for immunodominant secreted antigen 85B are the targets of MtrA, the essential response regulator
- PMID: 20223818
- PMCID: PMC2871449
- DOI: 10.1074/jbc.M109.040097
Mycobacterium tuberculosis origin of replication and the promoter for immunodominant secreted antigen 85B are the targets of MtrA, the essential response regulator
Abstract
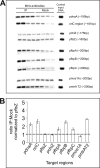
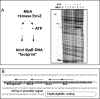
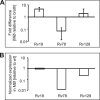
Efficient proliferation of Mycobacterium tuberculosis (Mtb) inside macrophage requires that the essential response regulator MtrA be optimally phosphorylated. However, the genomic targets of MtrA have not been identified. We show by chromatin immunoprecipitation and DNase I footprinting that the chromosomal origin of replication, oriC, and the promoter for the major secreted immunodominant antigen Ag85B, encoded by fbpB, are MtrA targets. DNase I footprinting analysis revealed that MtrA recognizes two direct repeats of GTCACAgcg-like sequences and that MtrA approximately P, the phosphorylated form of MtrA, binds preferentially to these targets. The oriC contains several MtrA motifs, and replacement of all motifs or of a single select motif with TATATA compromises the ability of oriC plasmids to maintain stable autonomous replication in wild type and MtrA-overproducing strains, indicating that the integrity of the MtrA motif is necessary for oriC replication. The expression of the fbpB gene is found to be down-regulated in Mtb cells upon infection when these cells overproduce wild type MtrA but not when they overproduce a nonphosphorylated MtrA, indicating that MtrA approximately P regulates fbpB expression. We propose that MtrA is a regulator of oriC replication and that the ability of MtrA to affect apparently unrelated targets, i.e. oriC and fbpB, reflects its main role as a coordinator between the proliferative and pathogenic functions of Mtb.
Figures




Similar articles
-
Mycobacterium tuberculosis oriC sequestration by MtrA response regulator.Mol Microbiol. 2015 Oct;98(3):586-604. doi: 10.1111/mmi.13144. Epub 2015 Aug 31. Mol Microbiol. 2015. PMID: 26207528 Free PMC article.
-
Mycobacterium tuberculosis mtrA merodiploid strains with point mutations in the signal-receiving domain of MtrA exhibit growth defects in nutrient broth.Plasmid. 2011 May;65(3):210-8. doi: 10.1016/j.plasmid.2011.01.002. Epub 2011 Feb 3. Plasmid. 2011. PMID: 21295603 Free PMC article.
-
Global mapping of MtrA-binding sites links MtrA to regulation of its targets in Mycobacterium tuberculosis.Microbiology (Reading). 2018 Jan;164(1):99-110. doi: 10.1099/mic.0.000585. Epub 2017 Nov 28. Microbiology (Reading). 2018. PMID: 29182512
-
Initiation of DNA Replication at the Chromosomal Origin of E. coli, oriC.Adv Exp Med Biol. 2017;1042:79-98. doi: 10.1007/978-981-10-6955-0_4. Adv Exp Med Biol. 2017. PMID: 29357054 Review.
-
How bacteria initiate DNA replication comes into focus.Bioessays. 2025 Jan;47(1):e2400151. doi: 10.1002/bies.202400151. Epub 2024 Oct 10. Bioessays. 2025. PMID: 39390825 Review.
Cited by
-
The orisome: structure and function.Front Microbiol. 2015 Jun 2;6:545. doi: 10.3389/fmicb.2015.00545. eCollection 2015. Front Microbiol. 2015. PMID: 26082765 Free PMC article. Review.
-
Recent Advances in Histidine Kinase-Targeted Antimicrobial Agents.Front Chem. 2022 Jul 4;10:866392. doi: 10.3389/fchem.2022.866392. eCollection 2022. Front Chem. 2022. PMID: 35860627 Free PMC article. Review.
-
Targeting multiple response regulators of Mycobacterium tuberculosis augments the host immune response to infection.Sci Rep. 2016 May 16;6:25851. doi: 10.1038/srep25851. Sci Rep. 2016. PMID: 27181265 Free PMC article.
-
Mutation of MtrA at the Predicted Phosphorylation Site Abrogates Its Role as a Global Regulator in Streptomyces venezuelae.Microbiol Spectr. 2022 Apr 27;10(2):e0213121. doi: 10.1128/spectrum.02131-21. Epub 2022 Mar 16. Microbiol Spectr. 2022. PMID: 35293797 Free PMC article.
-
Protein kinases PknA and PknB independently and coordinately regulate essential Mycobacterium tuberculosis physiologies and antimicrobial susceptibility.PLoS Pathog. 2020 Apr 7;16(4):e1008452. doi: 10.1371/journal.ppat.1008452. eCollection 2020 Apr. PLoS Pathog. 2020. PMID: 32255801 Free PMC article.
References
-
- North R. J., Jung Y. J. (2004) Annu. Rev. Immunol. 22, 599–623 - PubMed
-
- Cole S. T., Brosch R., Parkhill J., Garnier T., Churcher C., Harris D., Gordon S. V., Eiglmeier K., Gas S., Barry C. E., 3rd, Tekaia F., Badcock K., Basham D., Brown D., Chillingworth T., Connor R., Davies R., Devlin K., Feltwell T., Gentles S., Hamlin N., Holroyd S., Hornsby T., Jagels K., Krogh A., McLean J., Moule S., Murphy L., Oliver K., Osborne J., Quail M. A., Rajandream M. A., Rogers J., Rutter S., Seeger K., Skelton J., Squares R., Squares S., Sulston J. E., Taylor K., Whitehead S., Barrell B. G. (1998) Nature 393, 537–544 - PubMed
-
- Hoch J. A. (2000) Curr. Opin. Microbiol. 3, 165–170 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

