Multiple pathways for protein phosphatase 1 (PP1) regulation of Na-K-2Cl cotransporter (NKCC1) function: the N-terminal tail of the Na-K-2Cl cotransporter serves as a regulatory scaffold for Ste20-related proline/alanine-rich kinase (SPAK) AND PP1
- PMID: 20223824
- PMCID: PMC2863198
- DOI: 10.1074/jbc.M110.112672
Multiple pathways for protein phosphatase 1 (PP1) regulation of Na-K-2Cl cotransporter (NKCC1) function: the N-terminal tail of the Na-K-2Cl cotransporter serves as a regulatory scaffold for Ste20-related proline/alanine-rich kinase (SPAK) AND PP1
Abstract
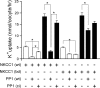
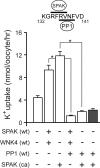
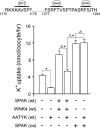
The Na-K-2Cl cotransporter (NKCC1) participates in epithelial transport and in cell volume maintenance by mediating the movement of ions and water across plasma membranes. Functional studies have previously demonstrated that NKCC1 activity is stimulated by protein phosphatase 1 (PP1) inhibitors. In this study, we utilized both in vivo (heterologous cRNA expression in Xenopus laevis oocytes) and in vitro ((32)P-phosphorylation assays with glutathione S-transferase fusion proteins) experiments to determine whether PP1 exerts its inhibitory effect directly on the cotransporter, or indirectly by affecting the activating kinase. We found that PP1 reduced NKCC1 activity in oocytes under both isotonic and hypertonic conditions to the same level as in water-injected controls. Interestingly, mutation of key residues in the PP1 binding motif located in the N-terminal tail of NKCC1 significantly reduced the inhibitory effect of PP1. In vitro experiments performed with recombinant PP1, SPAK (Ste20-related proline/alanine-rich kinase, which activates NKCC1), and the N terminus of NKCC1 fused to glutathione S-transferase demonstrated that PP1 dephosphorylated both the kinase and the cotransporter in a time-dependent manner. More importantly, PP1 dephosphorylation of SPAK was significantly greater when protein-protein interaction between the kinase and the N-terminal tail of NKCC1 was present in the reaction, indicating the necessity of scaffolding the phosphatase and kinase in proximity to one another. Taken together, our data are consistent with PP1 inhibiting NKCC1 activity directly by dephosphorylating the cotransporter and indirectly by dephosphorylating SPAK.
Figures
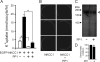
Similar articles
-
Apoptosis-associated tyrosine kinase scaffolding of protein phosphatase 1 and SPAK reveals a novel pathway for Na-K-2C1 cotransporter regulation.Am J Physiol Cell Physiol. 2007 May;292(5):C1809-15. doi: 10.1152/ajpcell.00580.2006. Epub 2007 Jan 31. Am J Physiol Cell Physiol. 2007. PMID: 17267545
-
A single binding motif is required for SPAK activation of the Na-K-2Cl cotransporter.Cell Physiol Biochem. 2007;20(1-4):131-42. doi: 10.1159/000104161. Cell Physiol Biochem. 2007. PMID: 17595523
-
Volume sensitivity of cation-Cl- cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4.Am J Physiol Cell Physiol. 2006 Jan;290(1):C134-42. doi: 10.1152/ajpcell.00037.2005. Epub 2005 Jun 1. Am J Physiol Cell Physiol. 2006. PMID: 15930150
-
Role of SPAK and OSR1 signalling in the regulation of NaCl cotransporters.Curr Opin Nephrol Hypertens. 2011 Sep;20(5):534-40. doi: 10.1097/MNH.0b013e3283484b06. Curr Opin Nephrol Hypertens. 2011. PMID: 21610494 Review.
-
SPAK and OSR1, key kinases involved in the regulation of chloride transport.Acta Physiol (Oxf). 2006 May-Jun;187(1-2):103-13. doi: 10.1111/j.1748-1716.2006.01565.x. Acta Physiol (Oxf). 2006. PMID: 16734747 Review.
Cited by
-
Kinase regulation of Na+-K+-2Cl- cotransport in primary afferent neurons.J Physiol. 2010 Sep 15;588(Pt 18):3365-73. doi: 10.1113/jphysiol.2010.190769. Epub 2010 May 24. J Physiol. 2010. PMID: 20498230 Free PMC article. Review.
-
Functional identification of protein phosphatase 1-binding consensus residues in NBCe1-B.Korean J Physiol Pharmacol. 2018 Jan;22(1):91-99. doi: 10.4196/kjpp.2018.22.1.91. Epub 2017 Dec 22. Korean J Physiol Pharmacol. 2018. PMID: 29302216 Free PMC article.
-
The WNK/SPAK and IRBIT/PP1 pathways in epithelial fluid and electrolyte transport.Physiology (Bethesda). 2012 Oct;27(5):291-9. doi: 10.1152/physiol.00028.2012. Physiology (Bethesda). 2012. PMID: 23026752 Free PMC article. Review.
-
Molecular physiology of SPAK and OSR1: two Ste20-related protein kinases regulating ion transport.Physiol Rev. 2012 Oct;92(4):1577-617. doi: 10.1152/physrev.00009.2012. Physiol Rev. 2012. PMID: 23073627 Free PMC article. Review.
-
Structural Pharmacology of Cation-Chloride Cotransporters.Membranes (Basel). 2022 Nov 29;12(12):1206. doi: 10.3390/membranes12121206. Membranes (Basel). 2022. PMID: 36557113 Free PMC article. Review.
References
-
- Russell J. M. (2000) Physiol. Rev. 80, 211–276 - PubMed
-
- Gamba G. (2005) Physiol. Rev. 85, 423–493 - PubMed
-
- Lytle C., Forbush B., 3rd (1992) J. Biol. Chem. 267, 25438–25443 - PubMed
-
- Lytle C. (1997) J. Biol. Chem. 272, 15069–15077 - PubMed
-
- Piechotta K., Lu J., Delpire E. (2002) J. Biol. Chem. 277, 50812–50819 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

