HPV testing on self collected cervicovaginal lavage specimens as screening method for women who do not attend cervical screening: cohort study
- PMID: 20223872
- PMCID: PMC2837143
- DOI: 10.1136/bmj.c1040
HPV testing on self collected cervicovaginal lavage specimens as screening method for women who do not attend cervical screening: cohort study
Erratum in
-
HPV testing on self collected cervicovaginal lavage specimens as screening method for women who do not attend cervical screening: cohort study.BMJ. 2016 May 18;353:i2823. doi: 10.1136/bmj.i2823. BMJ. 2016. PMID: 27193898 No abstract available.
Abstract
Objective: To determine whether offering self sampling of cervicovaginal material for high risk human papillomavirus (HPV) testing is an effective screening method for women who do not attend regular cervical screening programmes.
Design: Cohort study (the PROHTECT trial). Settings Noord-Holland and Flevoland regions of the Netherlands, December 2006 to December 2007, including 13 laboratories, gynaecologists, and more than 800 general practitioners.
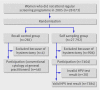
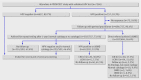
Participants: 28 073 women who had not responded to two invitations to the regular cervical screening programme: 27 792 women were assigned to the self sampling group and invited to submit a self collected cervicovaginal sample for HPV testing; 281 were assigned to the recall control group and received a second re-invitation for conventional cytology.
Intervention: Women with a positive result on the high risk HPV test on their self sample material were referred to their general practitioner. Women with abnormal results on cytology were referred for colposcopy. Women with normal results on cytology were re-evaluated after one year by cytology and high risk HPV testing and referred for colposcopy if either result was positive.
Main outcome measures: Attendance rate in both groups and yield of cervical intraepithelial neoplasia grade II/III or worse (>or=CIN II/>or=CIN III) in self sampling responders.
Results: The compliance rate in the self sampling group was significantly higher than in the control group (crude 26.6% v 16.4%, P<0.001; adjusted 27.5% v 16.6%, P<0.001). The number of detected >or=CIN II and >or=CIN III lesions in self sampling responders was 99 (1.3%) and 76 (1.0%), respectively. Self sampling responders who had not participated in the previous round of screening (43%) had increased relative risks of >or=CIN II (2.04, 95% confidence interval 1.27 to 3.28) and >or=CIN III (2.28, 1.31 to 3.96) compared with self sampling women who had been screened in the previous round (57%).
Conclusions: Offering self sampling by sending a device for collecting cervicovaginal specimens for high risk HPV testing to women who did not attend regular screening is a feasible and effective method of increasing coverage in a screening programme. The response rate and the yield of high grade lesions support implementation of this method for such women. Trial registration ISRCTN45527158.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures
Similar articles
-
Primary screening for high risk HPV by home obtained cervicovaginal lavage is an alternative screening tool for unscreened women.J Clin Pathol. 2002 Jun;55(6):435-9. doi: 10.1136/jcp.55.6.435. J Clin Pathol. 2002. PMID: 12037026 Free PMC article.
-
Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial.Lancet Oncol. 2012 Jan;13(1):78-88. doi: 10.1016/S1470-2045(11)70296-0. Epub 2011 Dec 14. Lancet Oncol. 2012. PMID: 22177579 Clinical Trial.
-
Age-specific evaluation of primary human papillomavirus screening vs conventional cytology in a randomized setting.J Natl Cancer Inst. 2009 Dec 2;101(23):1612-23. doi: 10.1093/jnci/djp367. Epub 2009 Nov 9. J Natl Cancer Inst. 2009. PMID: 19903804 Clinical Trial.
-
High-risk HPV testing on self-sampled versus clinician-collected specimens: a review on the clinical accuracy and impact on population attendance in cervical cancer screening.Int J Cancer. 2013 May 15;132(10):2223-36. doi: 10.1002/ijc.27790. Epub 2012 Sep 14. Int J Cancer. 2013. PMID: 22907569 Review.
-
Invitation strategy of vaginal HPV self-sampling to improve participation in cervical cancer screening: a systematic review and meta-analysis of randomized trials.BMC Public Health. 2024 Sep 10;24(1):2461. doi: 10.1186/s12889-024-19881-0. BMC Public Health. 2024. PMID: 39256726 Free PMC article.
Cited by
-
The indicating FTA elute cartridge a solid sample carrier to detect high-risk HPV and high-grade cervical lesions.J Mol Diagn. 2011 Jul;13(4):371-6. doi: 10.1016/j.jmoldx.2011.02.003. Epub 2011 Apr 29. J Mol Diagn. 2011. PMID: 21704269 Free PMC article.
-
Test performance and acceptability of self- versus provider-collected swabs for high-risk HPV DNA testing in female-to-male trans masculine patients.PLoS One. 2018 Mar 14;13(3):e0190172. doi: 10.1371/journal.pone.0190172. eCollection 2018. PLoS One. 2018. PMID: 29538411 Free PMC article. Clinical Trial.
-
Primary human papillomavirus testing by clinician- versus self-collection: Awareness and acceptance among cervical cancer screening-eligible women.J Med Screen. 2024 Dec;31(4):223-231. doi: 10.1177/09691413241260019. Epub 2024 Jun 13. J Med Screen. 2024. PMID: 38869176 Free PMC article.
-
Prevalence of Human Papillomavirus in Self-Taken Samples from Screening Nonattenders.J Clin Microbiol. 2017 Oct;55(10):2913-2923. doi: 10.1128/JCM.00550-17. Epub 2017 Jul 19. J Clin Microbiol. 2017. PMID: 28724554 Free PMC article.
-
Comparing the Costs and Diagnostic Outcomes of Replacing Cytology with the QIAsure DNA Methylation Test as a Triage within HPV Primary Cervical Cancer Screening in The Netherlands.Diagnostics (Basel). 2023 Dec 6;13(24):3612. doi: 10.3390/diagnostics13243612. Diagnostics (Basel). 2023. PMID: 38132196 Free PMC article.
References
-
- Bais AG, van Kemenade FJ, Berkhof J, Verheijen RH, Snijders PJ, Voorhorst F, et al. Human papillomavirus testing on self-sampled cervicovaginal brushes: an effective alternative to protect nonresponders in cervical screening programs. Int J Cancer 2007;120:1505-10. - PubMed
-
- Raab SS, Grzybicki DM, Zarbo RJ, Jensen C, Geyer SJ, Janosky JE, et al. Frequency and outcome of cervical cancer prevention failures in the United States. Am J Clin Pathol 2007;128:817-24. - PubMed
-
- Sawaya GF, Grimes DA. New technologies in cervical cytology screening: a word of caution. Obstet Gynecol 1999;94:307-10. - PubMed
-
- Sung HY, Kearney KA, Miller M, Kinney W, Sawaya GF, Hiatt RA. Papanicolaou smear history and diagnosis of invasive cervical carcinoma among members of a large prepaid health plan. Cancer 2000;88:2283-9. - PubMed
-
- Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet 2004;364:249-56. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical