Chemical enhancement of torsinA function in cell and animal models of torsion dystonia
- PMID: 20223934
- PMCID: PMC2860854
- DOI: 10.1242/dmm.003715
Chemical enhancement of torsinA function in cell and animal models of torsion dystonia
Abstract
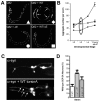
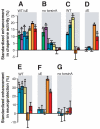
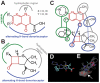
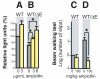
Movement disorders represent a significant societal burden for which therapeutic options are limited and focused on treating disease symptomality. Early-onset torsion dystonia (EOTD) is one such disorder characterized by sustained and involuntary muscle contractions that frequently cause repetitive movements or abnormal postures. Transmitted in an autosomal dominant manner with reduced penetrance, EOTD is caused in most cases by the deletion of a glutamic acid (DeltaE) in the DYT1 (also known as TOR1A) gene product, torsinA. Although some patients respond well to anticholingerics, therapy is primarily limited to either neurosurgery or chemodenervation. As mutant torsinA (DeltaE) expression results in decreased torsinA function, therapeutic strategies directed toward enhancement of wild-type (WT) torsinA activity in patients who are heterozygous for mutant DYT1 may restore normal cellular functionality. Here, we report results from the first-ever screen for candidate small molecule therapeutics for EOTD, using multiple activity-based readouts for torsinA function in Caenorhabditis elegans, subsequent validation in human DYT1 patient fibroblasts, and behavioral rescue in a mouse model of DYT1 dystonia. We exploited the nematode to rapidly discern chemical effectors of torsinA and identified two classes of antibiotics, quinolones and aminopenicillins, which enhance WT torsinA activity in two separate in vivo assays. Representative molecules were assayed in EOTD patient fibroblasts for improvements in torsinA-dependent secretory function, which was improved significantly by ampicillin. Furthermore, a behavioral defect associated with an EOTD mouse knock-in model was also rescued following administration of ampicillin. These combined data indicate that specific small molecules that enhance torsinA activity represent a promising new approach toward therapeutic development for EOTD, and potentially for other diseases involving the processing of mutant proteins.
Figures

Comment in
-
Invertebrate insights into autism.Dis Model Mech. 2010 Nov-Dec;3(11-12):665-6. doi: 10.1242/dmm.005876. Epub 2010 Aug 10. Dis Model Mech. 2010. PMID: 20699478 No abstract available.
References
-
- Allison PD. (2003). Logistic regression using the SAS system: theory and application. Cary, NC: SAS Institute, Inc
-
- Augood SJ, Penney JB, Friberg IK, Breakefield XO, Young AB, Ozelius LJ, Standaert DG. (1998). Expression of the early-onset torsion dystonia gene (DYT1) in human brain. Ann Neurol. 43, 669–673 - PubMed
-
- Basham SE, Rose LS. (2001). The Caenorhabditis elegans polarity gene ooc-5 encodes a torsin-related protein of the AAA ATPase superfamily. Development 128, 4645–4656 - PubMed
-
- Bragg DC, Camp SM, Kaufman CA, Wilbur JD, Boston H, Schuback DE, Hanson PI, Sena-Esteves M, Breakefield XO. (2004). Perinuclear biogenesis of mutant torsin-A inclusions in cultured cells infected with tetracycline-regulated herpes simplex virus type 1 amplicon vectors. Neuroscience 125, 651–661 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 NS047466/NS/NINDS NIH HHS/United States
- P30 NS057098/NS/NINDS NIH HHS/United States
- NS043176/NS/NINDS NIH HHS/United States
- NS047692/NS/NINDS NIH HHS/United States
- NS54246/NS/NINDS NIH HHS/United States
- NS57098/NS/NINDS NIH HHS/United States
- R21 NS065273/NS/NINDS NIH HHS/United States
- R21 NS047692/NS/NINDS NIH HHS/United States
- NS47466/NS/NINDS NIH HHS/United States
- R15 NS043176/NS/NINDS NIH HHS/United States
- P01 NS037409/NS/NINDS NIH HHS/United States
- NS037409/NS/NINDS NIH HHS/United States
- R01 NS054246/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

