Impaired organization and function of myofilaments in single muscle fibers from a mouse model of Pompe disease
- PMID: 20223998
- PMCID: PMC2867540
- DOI: 10.1152/japplphysiol.01253.2009
Impaired organization and function of myofilaments in single muscle fibers from a mouse model of Pompe disease
Abstract
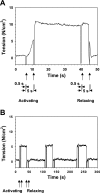
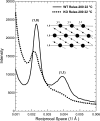
Pompe disease, a deficiency of lysosomal acid alpha-glucosidase, is a disorder of glycogen metabolism that can affect infants, children, or adults. In all forms of the disease, there is progressive muscle pathology leading to premature death. The pathology is characterized by accumulation of glycogen in lysosomes, autophagic buildup, and muscle atrophy. The purpose of the present investigation was to determine if myofibrillar dysfunction in Pompe disease contributes to muscle weakness beyond that attributed to atrophy. The study was performed on isolated myofibers dissected from severely affected fast glycolytic muscle in the alpha-glucosidase knockout mouse model. Psoas muscle fibers were first permeabilized, so that the contractile proteins could be directly relaxed or activated by control of the composition of the bathing solution. When normalized by cross-sectional area, single fibers from knockout mice produced 6.3 N/cm2 of maximum Ca2+-activated tension compared with 12.0 N/cm2 produced by wild-type fibers. The total protein concentration was slightly higher in the knockout mice, but concentrations of the contractile proteins myosin and actin remained unchanged. Structurally, X-ray diffraction showed that the actin and myosin filaments, normally arranged in hexagonal arrays, were disordered in the knockout muscle, and a lower fraction of myosin cross bridges was near the actin filaments in the relaxed muscle. The results are consistent with a disruption of actin and myosin interactions in the knockout muscles, demonstrating that impaired myofibrillar function contributes to weakness in the diseased muscle fibers.
Figures

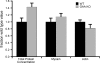
Similar articles
-
Suppression of mTORC1 activation in acid-α-glucosidase-deficient cells and mice is ameliorated by leucine supplementation.Am J Physiol Regul Integr Comp Physiol. 2014 Nov 15;307(10):R1251-9. doi: 10.1152/ajpregu.00212.2014. Epub 2014 Sep 17. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 25231351 Free PMC article.
-
Impaired performance of skeletal muscle in alpha-glucosidase knockout mice.Muscle Nerve. 2002 Jun;25(6):873-83. doi: 10.1002/mus.10125. Muscle Nerve. 2002. PMID: 12115977
-
Replacing acid alpha-glucosidase in Pompe disease: recombinant and transgenic enzymes are equipotent, but neither completely clears glycogen from type II muscle fibers.Mol Ther. 2005 Jan;11(1):48-56. doi: 10.1016/j.ymthe.2004.09.017. Mol Ther. 2005. PMID: 15585405
-
Autophagy in skeletal muscle: implications for Pompe disease.Int J Clin Pharmacol Ther. 2009;47 Suppl 1(Suppl 1):S42-7. doi: 10.5414/cpp47042. Int J Clin Pharmacol Ther. 2009. PMID: 20040311 Free PMC article. Review.
-
Pompe disease: current state of treatment modalities and animal models.Mol Genet Metab. 2007 Dec;92(4):299-307. doi: 10.1016/j.ymgme.2007.07.009. Epub 2007 Sep 7. Mol Genet Metab. 2007. PMID: 17826266 Review.
Cited by
-
Advancements in AAV-mediated Gene Therapy for Pompe Disease.J Neuromuscul Dis. 2020;7(1):15-31. doi: 10.3233/JND-190426. J Neuromuscul Dis. 2020. PMID: 31796685 Free PMC article. Review.
-
Intravenous Injection of an AAV-PHP.B Vector Encoding Human Acid α-Glucosidase Rescues Both Muscle and CNS Defects in Murine Pompe Disease.Mol Ther Methods Clin Dev. 2019 Jan 25;12:233-245. doi: 10.1016/j.omtm.2019.01.006. eCollection 2019 Mar 15. Mol Ther Methods Clin Dev. 2019. PMID: 30809555 Free PMC article.
-
Allele copy number and underlying pathology are associated with subclinical severity in equine type 1 polysaccharide storage myopathy (PSSM1).PLoS One. 2012;7(7):e42317. doi: 10.1371/journal.pone.0042317. Epub 2012 Jul 31. PLoS One. 2012. PMID: 22860112 Free PMC article.
-
Pros and cons of different ways to address dysfunctional autophagy in Pompe disease.Ann Transl Med. 2019 Jul;7(13):279. doi: 10.21037/atm.2019.03.51. Ann Transl Med. 2019. PMID: 31392191 Free PMC article. Review.
-
Three-dimensional tissue-engineered human skeletal muscle model of Pompe disease.Commun Biol. 2021 May 5;4(1):524. doi: 10.1038/s42003-021-02059-4. Commun Biol. 2021. PMID: 33953320 Free PMC article.
References
-
- Bahr BA. Lysosomal modulatory drugs for a broad strategy against protein accumulation disorders. Curr Alzheimer Res 6: 438–445, 2010 - PubMed
-
- Bijvoet AG, Van de Kamp EH, Kroos MA, Ding JH, Yang BZ, Visser P, Bakker CE, Verbeet MP, Oostra BA, Reuser AJ, Van der Ploeg AT. Generalized glycogen storage and cardiomegaly in a knockout mouse model of Pompe disease. Hum Mol Genet 7: 53–62, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

