Structural determinants in phycotoxins and AChBP conferring high affinity binding and nicotinic AChR antagonism
- PMID: 20224036
- PMCID: PMC2851920
- DOI: 10.1073/pnas.0912372107
Structural determinants in phycotoxins and AChBP conferring high affinity binding and nicotinic AChR antagonism
Abstract
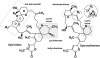
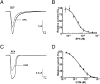
Spirolide and gymnodimine macrocyclic imine phycotoxins belong to an emerging class of chemical agents associated with marine algal blooms and shellfish toxicity. Analysis of 13-desmethyl spirolide C and gymnodimine A by binding and voltage-clamp recordings on muscle-type alpha1(2)betagammadelta and neuronal alpha3beta2 and alpha4beta2 nicotinic acetylcholine receptors reveals subnanomolar affinities, potent antagonism, and limited subtype selectivity. Their binding to acetylcholine-binding proteins (AChBP), as soluble receptor surrogates, exhibits picomolar affinities governed by diffusion-limited association and slow dissociation, accounting for apparent irreversibility. Crystal structures of the phycotoxins bound to Aplysia-AChBP ( approximately 2.4A) show toxins neatly imbedded within the nest of ar-omatic side chains contributed by loops C and F on opposing faces of the subunit interface, and which in physiological conditions accommodates acetylcholine. The structures also point to three major features: (i) the sequence-conserved loop C envelops the bound toxins to maximize surface complementarity; (ii) hydrogen bonding of the protonated imine nitrogen in the toxins with the carbonyl oxygen of loop C Trp147 tethers the toxin core centered within the pocket; and (iii) the spirolide bis-spiroacetal or gymnodimine tetrahydrofuran and their common cyclohexene-butyrolactone further anchor the toxins in apical and membrane directions, along the subunit interface. In contrast, the se-quence-variable loop F only sparingly contributes contact points to preserve the broad receptor subtype recognition unique to phycotoxins compared with other nicotinic antagonists. These data offer unique means for detecting spiroimine toxins in shellfish and identify distinctive ligands, functional determinants and binding regions for the design of new drugs able to target several receptor subtypes with high affinity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Marine Macrocyclic Imines, Pinnatoxins A and G: Structural Determinants and Functional Properties to Distinguish Neuronal α7 from Muscle α1(2)βγδ nAChRs.Structure. 2015 Jun 2;23(6):1106-15. doi: 10.1016/j.str.2015.04.009. Epub 2015 May 21. Structure. 2015. PMID: 26004441 Free PMC article.
-
The Cyclic Imine Core Common to the Marine Macrocyclic Toxins Is Sufficient to Dictate Nicotinic Acetylcholine Receptor Antagonism.Mar Drugs. 2024 Mar 27;22(4):149. doi: 10.3390/md22040149. Mar Drugs. 2024. PMID: 38667766 Free PMC article.
-
Cyclic imines: chemistry and mechanism of action: a review.Chem Res Toxicol. 2011 Nov 21;24(11):1817-29. doi: 10.1021/tx200182m. Epub 2011 Sep 9. Chem Res Toxicol. 2011. PMID: 21739960 Review.
-
Detection of gymnodimine-A and 13-desmethyl C spirolide phycotoxins by fluorescence polarization.Anal Chem. 2009 Apr 1;81(7):2708-14. doi: 10.1021/ac900144r. Anal Chem. 2009. PMID: 19278248
-
Cyclic imine toxins from dinoflagellates: a growing family of potent antagonists of the nicotinic acetylcholine receptors.J Neurochem. 2017 Aug;142 Suppl 2(Suppl 2):41-51. doi: 10.1111/jnc.13995. Epub 2017 Mar 21. J Neurochem. 2017. PMID: 28326551 Free PMC article. Review.
Cited by
-
Marine Macrocyclic Imines, Pinnatoxins A and G: Structural Determinants and Functional Properties to Distinguish Neuronal α7 from Muscle α1(2)βγδ nAChRs.Structure. 2015 Jun 2;23(6):1106-15. doi: 10.1016/j.str.2015.04.009. Epub 2015 May 21. Structure. 2015. PMID: 26004441 Free PMC article.
-
Natural compounds interacting with nicotinic acetylcholine receptors: from low-molecular weight ones to peptides and proteins.Toxins (Basel). 2015 May 14;7(5):1683-701. doi: 10.3390/toxins7051683. Toxins (Basel). 2015. PMID: 26008231 Free PMC article. Review.
-
Exhaustive Conformational Sampling of Complex Fused Ring Macrocycles Using Inverse Kinematics.J Chem Theory Comput. 2016 Sep 13;12(9):4674-87. doi: 10.1021/acs.jctc.6b00250. Epub 2016 Aug 4. J Chem Theory Comput. 2016. PMID: 27447193 Free PMC article.
-
Investigations into the toxicology of spirolides, a group of marine phycotoxins.Toxins (Basel). 2012 Jan;4(1):1-14. doi: 10.3390/toxins4010001. Epub 2011 Dec 30. Toxins (Basel). 2012. PMID: 22347619 Free PMC article.
-
Synthesis and biology of cyclic imine toxins, an emerging class of potent, globally distributed marine toxins.Nat Prod Rep. 2015 Mar;32(3):411-35. doi: 10.1039/c4np00089g. Nat Prod Rep. 2015. PMID: 25338021 Free PMC article. Review.
References
-
- Ciminiello P, Fattorusso E. Bivalve molluscs as vectors of marine biotoxins involved in seafood poisoning. Prog Mol Subcell Biol. 2006;43:53–82. - PubMed
-
- Gill S, et al. Neural injury biomarkers of novel shellfish toxins, spirolides: A pilot study using immunochemical and transcriptional analysis. Neurotoxicology. 2003;24:593–604. - PubMed
-
- O’Connor PD, Brimble MA. Synthesis of macrocyclic shellfish toxins containing spiroimine moieties. Nat Prod Rep. 2007;24:869–885. - PubMed
-
- Cembella AD, Lewis NI, Quilliam MA. Spirolide composition of micro-extracted pooled cells isolated from natural plankton assemblages and from cultures of the dinoflagellate Alexandrium ostenfeldii. Nat Toxins. 1999;7:197–206. - PubMed
-
- Hu T, et al. Characterization of spirolides a, c, and 13-desmethyl c, new marine toxins isolated from toxic plankton and contaminated shellfish. J Nat Prod. 2001;64:308–312. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

