Effector and regulatory B cells: modulators of CD4+ T cell immunity
- PMID: 20224569
- PMCID: PMC3038334
- DOI: 10.1038/nri2729
Effector and regulatory B cells: modulators of CD4+ T cell immunity
Abstract
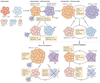
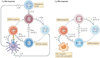
B cells are essential for humoral immunity, but the role that they have in regulating CD4(+) T cell responses remains controversial. However, new data showing that the transient depletion of B cells potently influences the induction, maintenance and reactivation of CD4(+) T cells, with the recent identification of antibody-independent functions of B cells, have reinvigorated interest in the many roles of B cells in both infectious and autoimmune diseases. In this Review, we discuss recent data showing how effector and regulatory B cells modulate CD4(+) T cell responses to pathogens and autoantigens.
Figures

References
-
- Constant S, Schweitzer N, West J, Ranney P, Bottomly K. B lymphocytes can be competent antigen-presenting cell for CD4+ T cells to protein antigens in vivo. J. Immunol. 1995;155:3734–3741. - PubMed
-
- Mastroeni P, Simmons C, Fowler R, Hormaeche CE, Dougan G. Igh-6−/− (B cell deficient) mice fail to mount solid acquired resistance to oral challenge with virulent Salmonella enterica Serovar Typhimurium and show impaired Th1 T-cell responses to Salmonella Antigens. Infect. Immun. 2000;68:46–53. - PMC - PubMed
-
- Bergmann CC, Ramakrishna C, Kornacki M, Stohlman SA. Impaired T cell immunity in B cell-deficient mice following viral central nervous system infection. J Immunol. 2001;167:1575–1583. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

