Efficacy and safety of capecitabine in combination with docetaxel and mitomycin C in patients with pre-treated pancreatic, gallbladder, and bile duct carcinoma
- PMID: 20224968
- PMCID: PMC11827915
- DOI: 10.1007/s00432-010-0843-6
Efficacy and safety of capecitabine in combination with docetaxel and mitomycin C in patients with pre-treated pancreatic, gallbladder, and bile duct carcinoma
Abstract
Purpose: Preclinical data indicate the improvement of the antitumor activity of capecitabine by mitomycin C and docetaxel through upregulation of thymidine phosphorylase activity. Therefore, we have established a combination regimen of these drugs (DocMitoCape), which demonstrated preliminary activity especially in bile duct and pancreatic carcinoma.
Methods: Here we report the safety and efficacy of the DocMitoCape regimen in pre-treated patients with gallbladder, bile duct, or pancreatic carcinoma. Treatment consisted of capecitabine (2,000 mg/m(2) days 1-14) in combination with docetaxel (40 mg/m(2) day 1) and mitomycin C (4 mg/m(2) day 1). Cycles were repeated on day 22. Toxicity was graded according to NCI-CTC criteria, and the antitumor activity was assessed by RECIST criteria.
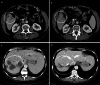
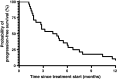
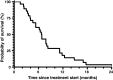
Results: Twenty-eight pre-treated patients with a median age of 59 suffering from pancreatic, gallbladder, intra- (IHCCC) or extrahepatic (EHCCC) bile duct carcinoma were included. Eleven patients had received ≥2 lines of prior chemotherapy. A total of 183 and a median of six cycles were administered (range 1-21). The mean dose intensity was as follows (cycles 1-2/3-4; %): capecitabine 97/92, docetaxel 100/100, mitomycin C 99/100. Main adverse events grades 2/3/4 were (n): leukocytopenia 3/2/2, anemia 13/4/0, thrombocytopenia 3/1/0, nausea/vomiting 2/1/0, diarrhea 5/1/0, hand-foot-skin reaction 7/0/0. Six patients achieved partial and seven patients minor remissions, while six patients had stable disease adding to a tumor control rate of 68%. Median progression-free and overall survival was 4.5 (range 1.0-44.9) and 6.8 months (range 1.5-44.9), respectively, calculated from the start of treatment.
Conclusion: In all, the DocMitoCape regimen exhibited a favorable safety profile and a high rate of tumor stabilizations in patients with pre-treated gallbladder, bile duct and pancreatic carcinoma. It might be considered after failure of standard regimens in these types of cancer.
Figures
References
-
- Chun JH, Kim HK, Lee JS, Choi JY, Hwangbo B, Lee HG, Park SR, Choi IJ, Kim CG, Ryu KW, Kim YW, Bae JM (2005) Weekly docetaxel in combination with capecitabine in patients with metastatic gastric cancer. Am J Clin Oncol 28:188–194 - PubMed
-
- Ferrero JM, Chamorey E, Oudard S, Dides S, Lesbats G, Cavaglione G, Nouyrigat P, Foa C, Kaphan R (2006) Phase II trial evaluating a docetaxel-capecitabine combination as treatment for hormone-refractory prostate cancer. Cancer 107:738–745 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

