Src-like adaptor protein regulates osteoclast generation and survival
- PMID: 20225239
- PMCID: PMC3680130
- DOI: 10.1002/jcb.22527
Src-like adaptor protein regulates osteoclast generation and survival
Abstract
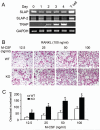
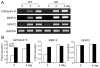
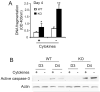
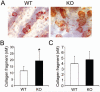
Src-like adaptor protein (SLAP) is a hematopoietic adaptor containing Src homology (SH)3 and SH2 motifs and a unique carboxy terminus. Unlike c-Src, SLAP lacks a tyrosine kinase domain. We investigated the role of SLAP in osteoclast development and resorptive function. Employing SLAP-deficient mice, we find lack of the adaptor enhances in vitro proliferation of osteoclast precursors in the form of bone marrow macrophages (BMMs), without altering their survival. Furthermore, osteoclastogenic markers appear more rapidly in SLAP-/- BMMs exposed to RANK ligand (RANKL). The accelerated proliferation of M-CSF-treated, SLAP-deficient precursors is associated with enhanced ERK activation. SLAP's role as a mediator of M-CSF signaling, in osteoclastic cells, is buttressed by complexing of the adaptor protein and c-Fms in lipid rafts. Unlike c-Src, SLAP does not impact resorptive function of mature osteoclasts but induces their early apoptosis. Thus, SLAP negatively regulates differentiation of osteoclasts and proliferation of their precursors. Conversely, SLAP decreases osteoclast death by inhibiting activation of caspase 3. These counterbalancing events yield indistinguishable bones of WT and SLAP-/- mice which contain equal numbers of osteoclasts in basal and stimulated conditions.
(c) 2010 Wiley-Liss, Inc.
Figures



Similar articles
-
Docosahexaenoic acid signaling attenuates the proliferation and differentiation of bone marrow-derived osteoclast precursors and promotes apoptosis in mature osteoclasts.Cell Signal. 2017 Jan;29:226-232. doi: 10.1016/j.cellsig.2016.11.007. Epub 2016 Nov 9. Cell Signal. 2017. PMID: 27836739
-
DAP12 couples c-Fms activation to the osteoclast cytoskeleton by recruitment of Syk.Mol Cell. 2008 Aug 8;31(3):422-31. doi: 10.1016/j.molcel.2008.06.023. Mol Cell. 2008. PMID: 18691974 Free PMC article.
-
A potential role for the Src-like adapter protein SLAP-2 in signaling by the colony stimulating factor-1 receptor.FEBS J. 2006 Apr;273(8):1791-804. doi: 10.1111/j.1742-4658.2006.05199.x. FEBS J. 2006. PMID: 16623714
-
Role of c-Src in cellular events associated with colony-stimulating factor-1-induced spreading in osteoclasts.Mol Reprod Dev. 1997 Jan;46(1):104-8. doi: 10.1002/(SICI)1098-2795(199701)46:1<104::AID-MRD16>3.0.CO;2-2. Mol Reprod Dev. 1997. PMID: 8981371 Review.
-
RTK SLAP down: the emerging role of Src-like adaptor protein as a key player in receptor tyrosine kinase signaling.Cell Signal. 2015 Feb;27(2):267-74. doi: 10.1016/j.cellsig.2014.11.010. Epub 2014 Nov 18. Cell Signal. 2015. PMID: 25446260 Review.
Cited by
-
Integrin-associated molecules and signalling cross talking in osteoclast cytoskeleton regulation.J Cell Mol Med. 2020 Mar;24(6):3271-3281. doi: 10.1111/jcmm.15052. Epub 2020 Feb 11. J Cell Mol Med. 2020. PMID: 32045092 Free PMC article. Review.
-
The Emerging and Diverse Roles of Src-Like Adaptor Proteins in Health and Disease.Mediators Inflamm. 2015;2015:952536. doi: 10.1155/2015/952536. Epub 2015 Aug 3. Mediators Inflamm. 2015. PMID: 26339145 Free PMC article. Review.
-
Dexamethasone Conjugates: Synthetic Approaches and Medical Prospects.Biomedicines. 2021 Mar 27;9(4):341. doi: 10.3390/biomedicines9040341. Biomedicines. 2021. PMID: 33801776 Free PMC article. Review.
-
Role of SRC-like adaptor protein (SLAP) in immune and malignant cell signaling.Cell Mol Life Sci. 2015 Jul;72(13):2535-44. doi: 10.1007/s00018-015-1882-6. Epub 2015 Mar 13. Cell Mol Life Sci. 2015. PMID: 25772501 Free PMC article. Review.
-
Acute heat stress induces differential gene expressions in the testes of a broiler-type strain of Taiwan country chickens.PLoS One. 2015 May 1;10(5):e0125816. doi: 10.1371/journal.pone.0125816. eCollection 2015. PLoS One. 2015. PMID: 25932638 Free PMC article.
References
-
- Akiyama T, Bouillet P, Miyazaki T, Kadono Y, Chikuda H, Chung UI, Fukuda A, Hikita A, Seto H, Okada T, Inaba T, Sanjay A, Baron R, Kawaguchi H, Oda H, Nakamura K, Strasser A, Tanaka S. Regulation of osteoclast apoptosis by ubiquitylation of proapoptotic BH3-only Bcl-2 family member Bim. EMBO J. 2003;22:6653–6664. - PMC - PubMed
-
- Alonso G, Koegl M, Mazurenko N, Courtneidge SA. Sequence requirements for binding of Src family tyrosine kinases to activated growth factor receptors. J Biol Chem. 1995;270:9840–9848. - PubMed
-
- Bourette RP, De Sepulveda P, Arnaud S, Dubreuil P, Rottapel R, Mouchiroud G. Suppressor of cytokine signaling 1 interacts with the macrophage colony-stimulating factor receptor and negatively regulates its proliferation signal. J Biol Chem. 2001;276:22133–22139. - PubMed
-
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

