Na(v)1.6a is required for normal activation of motor circuits normally excited by tactile stimulation
- PMID: 20225246
- PMCID: PMC2900195
- DOI: 10.1002/dneu.20791
Na(v)1.6a is required for normal activation of motor circuits normally excited by tactile stimulation
Abstract
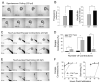
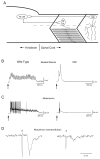
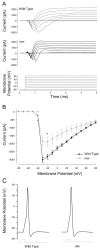
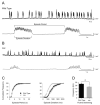
A screen for zebrafish motor mutants identified two noncomplementing alleles of a recessive mutation that were named non-active (nav(mi89) and nav(mi130)). nav embryos displayed diminished spontaneous and touch-evoked escape behaviors during the first 3 days of development. Genetic mapping identified the gene encoding Na(V)1.6a (scn8aa) as a potential candidate for nav. Subsequent cloning of scn8aa from the two alleles of nav uncovered two missense mutations in Na(V)1.6a that eliminated channel activity when assayed heterologously. Furthermore, the injection of RNA encoding wild-type scn8aa rescued the nav mutant phenotype indicating that scn8aa was the causative gene of nav. In-vivo electrophysiological analysis of the touch-evoked escape circuit indicated that voltage-dependent inward current was decreased in mechanosensory neurons in mutants, but they were able to fire action potentials. Furthermore, tactile stimulation of mutants activated some neurons downstream of mechanosensory neurons but failed to activate the swim locomotor circuit in accord with the behavioral response of initial escape contractions but no swimming. Thus, mutant mechanosensory neurons appeared to respond to tactile stimulation but failed to initiate swimming. Interestingly fictive swimming could be initiated pharmacologically suggesting that a swim circuit was present in mutants. These results suggested that Na(V)1.6a was required for touch-induced activation of the swim locomotor network.
Figures

Similar articles
-
Touch responsiveness in zebrafish requires voltage-gated calcium channel 2.1b.J Neurophysiol. 2012 Jul;108(1):148-59. doi: 10.1152/jn.00839.2011. Epub 2012 Apr 4. J Neurophysiol. 2012. PMID: 22490555 Free PMC article.
-
Zebrafish touch-insensitive mutants reveal an essential role for the developmental regulation of sodium current.J Neurosci. 1998 Nov 15;18(22):9181-91. doi: 10.1523/JNEUROSCI.18-22-09181.1998. J Neurosci. 1998. PMID: 9801358 Free PMC article.
-
touché Is required for touch-evoked generator potentials within vertebrate sensory neurons.J Neurosci. 2010 Jul 14;30(28):9359-67. doi: 10.1523/JNEUROSCI.1639-10.2010. J Neurosci. 2010. PMID: 20631165 Free PMC article.
-
Development of the locomotor network in zebrafish.Prog Neurobiol. 2002 Oct;68(2):85-111. doi: 10.1016/s0301-0082(02)00075-8. Prog Neurobiol. 2002. PMID: 12450489 Review.
-
Trafficking mechanisms underlying Nav channel subcellular localization in neurons.Channels (Austin). 2020 Dec;14(1):1-17. doi: 10.1080/19336950.2019.1700082. Channels (Austin). 2020. PMID: 31841065 Free PMC article. Review.
Cited by
-
Touch responsiveness in zebrafish requires voltage-gated calcium channel 2.1b.J Neurophysiol. 2012 Jul;108(1):148-59. doi: 10.1152/jn.00839.2011. Epub 2012 Apr 4. J Neurophysiol. 2012. PMID: 22490555 Free PMC article.
-
pigk Mutation underlies macho behavior and affects Rohon-Beard cell excitability.J Neurophysiol. 2015 Aug;114(2):1146-57. doi: 10.1152/jn.00355.2015. Epub 2015 Jul 1. J Neurophysiol. 2015. PMID: 26133798 Free PMC article.
-
Effective sensory modality activating an escape triggering neuron switches during early development in zebrafish.J Neurosci. 2012 Apr 25;32(17):5810-20. doi: 10.1523/JNEUROSCI.6169-11.2012. J Neurosci. 2012. PMID: 22539843 Free PMC article.
-
Zebrafish sin3b mutants are viable but have size, skeletal, and locomotor defects.Dev Dyn. 2017 Nov;246(11):946-955. doi: 10.1002/dvdy.24581. Epub 2017 Sep 25. Dev Dyn. 2017. PMID: 28850761 Free PMC article.
-
TRPM7 is required within zebrafish sensory neurons for the activation of touch-evoked escape behaviors.J Neurosci. 2011 Aug 10;31(32):11633-44. doi: 10.1523/JNEUROSCI.4950-10.2011. J Neurosci. 2011. PMID: 21832193 Free PMC article.
References
-
- Beattie CE, Hatta K, Halpern ME, Liu H, Eisen JS, Kimmel CB. Temporal separation in the specification of primary and secondary motoneurons in zebrafish. Dev Biol. 1997;187:171–182. - PubMed
-
- Buss RR, Drapeau P. Activation of embryonic red and white muscle fibers during fictive swimming in the developing zebrafish. J Neurophysiol. 2002;87:1244–1251. - PubMed
-
- Buss RR, Drapeau P. Physiological properties of zebrafish embryonic red and white muscle fibers during early development. J Neurophysiology. 2000;84:1545–1557. - PubMed
-
- Buss RR, Drapeau P. Synaptic drive to motoneurons during fictive swimming in the developing zebrafish. J Neurophysiol. 2001;86:197–210. - PubMed
-
- Chandrasekhar A, Shcauerte HE, Haffter P, Kuwada JY. The zebrafish detour gene is essential for cranial but not spinal motor neuron induction. Development. 1999;126:2727–2737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

